Belinostat (PXD101)
|
- ₹0
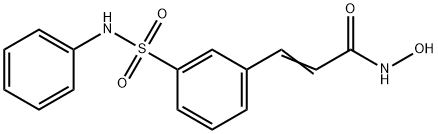
- Product name: Belinostat (PXD101)
- CAS: 414864-00-9
- MF: C15H14N2O4S
- MW: 318.35
- EINECS:
- MDL Number:MFCD08064035
- Synonyms:PXD101, Beliomsta;BELINOSTAT (PXD101);PXD101;N-HYDROXY-3-[3-[(PHENYLAMINO)SULFONYL]PHENYL]-2-PROPENAMIDE;N-HYDROXY-3-(3-PHENYLSULFAMOYLPHENYL)ACRYLAMIDE;Belinostat(PXD101);2-PropenaMide, N-hydroxy-3-[3-[(phenylaMino)sulfonyl]phenyl]-;PX105684;Belinostat-13C6
| Manufacturer | Product number | Product description | Packaging | Price | Updated | Buy |
|---|
Properties
Melting point :142 - 145°C
Density :1.427±0.06 g/cm3(Predicted)
storage temp. :Amber Vial, Refrigerator, Under inert atmosphere
solubility :DMSO (Slightly), Methanol (Slightly)
form :Solid
pka :8.31±0.10(Predicted)
color :Off-White to Pale Orange
Stability :Light Sensitive
Density :1.427±0.06 g/cm3(Predicted)
storage temp. :Amber Vial, Refrigerator, Under inert atmosphere
solubility :DMSO (Slightly), Methanol (Slightly)
form :Solid
pka :8.31±0.10(Predicted)
color :Off-White to Pale Orange
Stability :Light Sensitive
Safety Information
| Symbol(GHS): | ||||||||
|---|---|---|---|---|---|---|---|---|
| Signal word: | ||||||||
| Hazard statements: |
|
|||||||
| Precautionary statements: |
|
Description
Belinostat is a drug which was developed by Spectrum Pharmaceuticals and is currently marketed by Onxeo as Beleodaq®. The drug, which received fast track designation by the United States Food and Drug Administration (US FDA) and was approved for the treatment of hematological malignancies and solid tumors associated with peripheral T-cell lymphoma (PTCL) in 2014, is a histone deacetylase (HDAC) inhibitor and is the third such treatment to receive accelerated approval for PTCL, the others being vorinostat (Zolinza®) and pralatrexate (Folotyn®). Although belinostat was not yet approved in Europe as of August 2014, the compound exhibits a safety profile considered to be acceptable for HDAC inhibitors–less than 25% of patients reported adverse effects and these most frequently were nausea, fatigue, pyrexia, anemia, and emesis.Related product price
- Entinostat
₹41373.15-122387.45 - Vorinostat
₹10110.55-40983.45 - PXD-101
₹6600-36707.58






