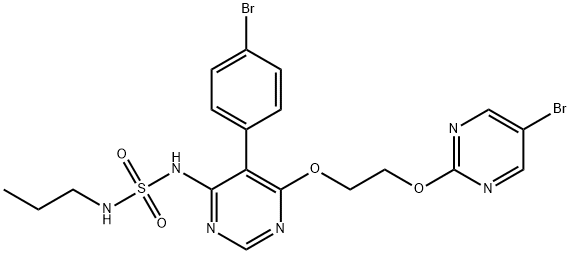
macitentan
|
- ₹0
- Product name: macitentan
- CAS: 441798-33-0
- MF: C19H20Br2N6O4S
- MW: 588.27
- EINECS:1308068-626-2
- MDL Number:MFCD17167076
- Synonyms:N-[5-(4-Bromophenyl)-6-[2-[(5-bromo-2-pyrimidinyl)-oxy]ethoxy]-4-pyrimidinyl]-N'-propylsulfami;macitentan;N-[5-(4-Bromophenyl)-6-[2-[(5-bromo-2-pyrimidinyl)oxy]ethoxy]-4-pyrimidinyl]-N'-propylsulfamide;ACT 064992;ACT-064992;ACT-064992 / ACT064992;Macitentan, >=99%;SulfaMide,N-[5-(4-broMophenyl)-6-[2-[(5-broMo-2-pyriMidinyl)oxy]ethoxy]-4-pyriMidinyl]-N'-propyl-
| Manufacturer | Product number | Product description | Packaging | Price | Updated | Buy |
|---|
Properties
Boiling point :692.4±65.0 °C(Predicted)
Density :1.675
storage temp. :Store at -20°C
solubility :≥24.4 mg/mL in DMSO; insoluble in H2O; insoluble in EtOH
form :solid
pka :4.99±0.50(Predicted)
color :White to off-white
InChI :InChI=1S/C19H20Br2N6O4S/c1-2-7-26-32(28,29)27-17-16(13-3-5-14(20)6-4-13)18(25-12-24-17)30-8-9-31-19-22-10-15(21)11-23-19/h3-6,10-12,26H,2,7-9H2,1H3,(H,24,25,27)
InChIKey :JGCMEBMXRHSZKX-UHFFFAOYSA-N
SMILES :S(NC1C(C2=CC=C(Br)C=C2)=C(OCCOC2=NC=C(Br)C=N2)N=CN=1)(NCCC)(=O)=O
Density :1.675
storage temp. :Store at -20°C
solubility :≥24.4 mg/mL in DMSO; insoluble in H2O; insoluble in EtOH
form :solid
pka :4.99±0.50(Predicted)
color :White to off-white
InChI :InChI=1S/C19H20Br2N6O4S/c1-2-7-26-32(28,29)27-17-16(13-3-5-14(20)6-4-13)18(25-12-24-17)30-8-9-31-19-22-10-15(21)11-23-19/h3-6,10-12,26H,2,7-9H2,1H3,(H,24,25,27)
InChIKey :JGCMEBMXRHSZKX-UHFFFAOYSA-N
SMILES :S(NC1C(C2=CC=C(Br)C=C2)=C(OCCOC2=NC=C(Br)C=N2)N=CN=1)(NCCC)(=O)=O
Safety Information
| Symbol(GHS): |

|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Signal word: | Danger | ||||||||||||||
| Hazard statements: |
|
||||||||||||||
| Precautionary statements: |
|
Description
Macitentan (also known as ACT-064992) received US FDA approval in October 2013 for the treatment of pulmonary arterial hypertension (PAH) (WHO group I) to delay disease progression. Treatment options include phosphodiesterase type 5 inhibitors, prostacyclins, and the endothelin receptor antagonists bosentan and ambrisentan. Macitentan was discovered through SAR studies starting with the bosentan structure with three main goals: (1) to increase potency for both endothelin receptor A and B (ETA and ETB) subtypes; (2) to improve tissue distribution to reach the target receptors; and (3) to avoid bile salt transport inhibition. Starting with the bosentan sulfonamido-pyrimidinyl central core, potency was increased 10-fold via incorporation of a bromopyrimidinyl ethylene glycol ether, as found in the clinical endothelin antagonist, T-0201. An aryl ether in bosentan was replaced with the bromophenyl group in macitentan, and a substituent on the 2-position of the central pyrimidine was replaced with hydrogen. Several sulfonamides and alkyl sulfamates were explored, with the propylsulfamate providing the best combination of in vitro potency, especially for ETB antagonism, and in vivo efficacy.Related product price
- Rapamycin
₹4194-370089 - Sitaxentan sodium
₹12372.98-49697.58 - Fingolimod hydrochloride
₹7068.73-100932.3






