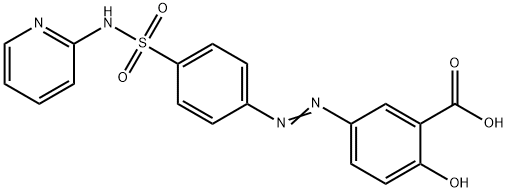
Sulfasalazine
- CAS No.
- 599-79-1
- Chemical Name:
- Sulfasalazine
- Synonyms
- sasp;salicylazosulfapyridine;sulphasalazine;SSZ;azulfidine;sulfasalazin;SALAZOSULFAPYRIDINE;s.a.s.-500;salazopyrin;si-88
- CBNumber:
- CB0181156
- Molecular Formula:
- C18H14N4O5S
- Molecular Weight:
- 398.39
- MOL File:
- 599-79-1.mol
- Modify Date:
- 2024/8/1 13:52:05
| Melting point | 260-265 °C (dec.)(lit.) |
|---|---|
| Boiling point | 689.3±65.0 °C(Predicted) |
| Density | 1.3742 (rough estimate) |
| refractive index | 1.6000 (estimate) |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | NH4OH 1 M: 50 mg/mL, clear, red |
| pka | pKa 0.6(H2O t = 20 I < 0.001) (Uncertain);2.4(H2O t = 20 I < 0.001) (Uncertain);9.7(H2O t = 20 I < 0.001) (Uncertain);11.8(H2O t = 20 I < 0.001) (Uncertain) |
| form | powder |
| color | Orange |
| Water Solubility | <0.1 g/100 mL at 25 ºC |
| Merck | 14,8942 |
| BRN | 356241 |
| BCS Class | 2,4 |
| Stability | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20° for up to 1 month. |
| InChIKey | NCEXYHBECQHGNR-QZQOTICOSA-N |
| CAS DataBase Reference | 599-79-1(CAS DataBase Reference) |
| IARC | 2B (Vol. 108) 2016 |
| EPA Substance Registry System | Salicylazosulfapyridine (599-79-1) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H317-H334 | |||||||||
| Precautionary statements | P261-P272-P280-P284-P302+P352-P333+P313 | |||||||||
| Hazard Codes | Xn | |||||||||
| Risk Statements | 42/43 | |||||||||
| Safety Statements | 22-29/56-45 | |||||||||
| RIDADR | 3077 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | VO6250000 | |||||||||
| F | 8 | |||||||||
| HS Code | 29350090 | |||||||||
| Toxicity | LD50 oral in rabbit: > 7500mg/kg | |||||||||
| NFPA 704 |
|
Sulfasalazine price More Price(4)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | S0883 | Sulfasalazine 97.0-101.5% | 599-79-1 | 10G | ₹8638.35 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | S0883 | Sulfasalazine 97.0-101.5% | 599-79-1 | 50G | ₹21455.15 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | S0883 | Sulfasalazine 97.0-101.5% | 599-79-1 | 100G | ₹35105.48 | 2022-06-14 | Buy |
| TCI Chemicals (India) | S0580 | Sulfasalazine | 599-79-1 | 25G | ₹7700 | 2022-05-26 | Buy |
Sulfasalazine Chemical Properties,Uses,Production
Description
Sulfasalazine (brand name Azulfidine in the U.S., Salazopyrin and Sulazine in Europe and Hong Kong) was developed in the 1950s specifically to treat rheumatoid arthritis. It was believed at the time that bacterial infections were the cause of rheumatoid arthritis. Sulfasalazine is a sulfa drug, (a derivative of mesalazine) and is formed by combining sulfa pyridine and salicylate with an azo bond. It may be abbreviated SSZ.
Chemical Properties
Brownish-Yellow Crystals
Uses
Sulfasalazine is an anti-inflammatory (gastrointestinal). Sulfasalazine has been used in granulomatous colitis.
Indications
Sulfasalazine is used in the treatment of inflammatory bowel disease, including ulcerative colitis and Crohn's disease. It is also indicated for use in rheumatoid arthritis and used in other types of inflammatory arthritis (e.g. psoriatic arthritis) where it has a beneficial effect. It is often well tolerated compared to other DMARDS.
In clinical trials for the treatment of chronic alcoholics, sulfasalazine has been found to reverse the scarring associated with cirrhosis of the liver .
Cells called myofibroblasts, which contribute to scar tissue in a diseased liver, also appear to secrete proteins that prevent the breakdown of the scar tissue. Sulfasalazine appears to retard this secretion.
Definition
ChEBI: An azobenzene consisting of diphenyldiazene having a carboxy substituent at the 4-position, a hydroxy substituent at the 3-position and a 2-pyridylaminosulphonyl substituent at the 4'-position.
General Description
Odorless yellow or brownish-yellow to orange powder. Tasteless.
Air & Water Reactions
Light sensitive and may be sensitive to prolonged exposure to air. Dust can be explosive when suspended in air at specific concentrations. Insoluble in water.
Fire Hazard
Flash point data for Salicylazosulfapyridine are not available; however, Salicylazosulfapyridine is probably combustible.
Pharmaceutical Applications
One of the earliest and most successful sulfonamides to be
developed was sulfapyridine, which fell into disuse because
of unwanted effects such as crystalluria. Later, a number of
salicylazosulfonamides, developed because of their increased
water solubility, showed anti-inflammatory properties; one of
them, sulfasalazine (salicylazosulfapyridine), has come into
general use for ulcerative colitis.
After oral administration, some intact compound is
absorbed from the upper gastrointestinal tract, appearing in
the blood in 1–2 h, but most is cleaved by colonic bacteria
to yield sulfapyridine and 5-aminosalicylic acid (mesalamine,
mesalazine). Controlled trials have confirmed the efficacy of
5-aminosalicylic acid alone in ulcerative colitis, the sulfonamide
component merely acting as a carrier. Thus, in remarkable
extension of the good fortune that attended the discovery
of sulfanilamide as the unexpected active principle of Prontosil, a cleavage product appears to be responsible for
the beneficial effect of sulfasalazine. Since most of the side
effects associated with sulfasalazine are attributable to sulfapyridine,
there seems little reason, other than cost, to use it
in preference to mesalamine.
Sulfasalazine is also of benefit in Crohn’s disease and rheumatoid
arthritis, but the role, if any, of sulfapyridine in the
overall effect is unclear.
Mechanism of action
Sulfasalazine is composed of sulfapyridine and 5- ASA molecules linked by an azo bond. Sulfapyridine has no effect on the inflammatory bowel disease, and instillation of this agent into the colon does not heal colonic mucosa.
Pharmacokinetics
sulfasalazine is poorly absorbed, with approximately 20% of the ingested sulfasalazine reaching the systemic circulation. The remainder of the ingested dose is metabolized by colonic bacteria into its components, sulfapyridine and mesalamine (5-ASA). Most of the sulfapyridine metabolized from sulfasalazine (60–80%) is absorbed in the colon following oral administration, and approximately 25% of the 5-ASA metabolized from sulfasalazine is absorbed in the colon.
Pharmacology
Sulfasalazine is a prodrug of which 70% is converted by colon bacteria to two active metabolites, sulfapyridine and 5-aminosalicylic acid (mesalamine). Sulfapyridine has antibacterial activities, and 5-aminosalicylic acid is antiinflammatory; however, these effects do not account for the ability of this drug to slow the processes of rheumatoid arthritis. Recent research suggests additional activities of sulfasalazine that may be relevant to these effects: its ability to increase adenosine levels, its inhibitory effects on IL-1 and TNF- release, and its inhibition of NF-κB.
Clinical Use
Sulfasalazine (2-hydroxy-5[[4-[(2-pyridinylamino)sulfonyl]phenyl]azo]benzoic acid or 5-[p-(2-pyridylsulfamoyl)phenylazo]salicylic acid) is a brownish yellow, odorlesspowder, slightly soluble in alcohol but practically insolublein water, ether, and benzene.
Sulfasalazine is broken down in the body to m-aminosalicylicacid and sulfapyridine. The drug is excreted throughthe kidneys and is detectable colorimetrically in the urine,producing an orange-yellow color when the urine is alkalineand no color when the urine is acid.
Side effects
Sulfsalazine metabolizes to sulfa pyridine. Serum levels should be monitored every three months, and more frequently at the outset. Serum levels above 50 μg / l are associated with side effects. In rare cases, Sulfasalazine can cause severe depression in young males. It can also cause temporary infertility. Immune thrombocytopenia has been reported.
Sulfasalazine inhibits dihydrofolate reductase, and can cause folate deficiency and megaloblastic anemia.
Sulfasalazine can cause hemolytic anemia in people with G6PD deficiency.
Precautions
Sulfasalazine is contraindicated in individuals with hypersensitivityto salicylates, sulfonamides, sulfonylureas,and certain diuretics (furosemide, thiazides, andcarbonic anhydrase inhibitors). Because it can causekernicterus, sulfasalazine is contraindicated in infantsand children under 2 years of age. Sulfasalazine passesinto breast milk and is therefore contraindicated fornursing mothers. Similarly, pregnant women near termshould not use this drug, although it appears to be thesafest of the DMARDs during early pregnancy.Sulfasalazine can precipitate attacks of porphyria andshould not be used by individuals with bowel or urinaryobstruction.
Sulfasalazine can inhibit the absorption of cardiacglycosides and folic acid. It may displace certain drugs,including warfarin, phenytoin, methotrexate, tolbutamide,chlorpropamide, and oral sulfonylureas, fromtheir protein binding sites. Sulfasalazine can diminishthe effectiveness of penicillins and estrogen-containingoral contraceptives.
Sulfasalazine Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| GLP Pharma Standards | +91 9866074638 | Hyderabad, India | 1644 | 58 | Inquiry |
| Virchow Groups | +919394860022 | Telangana, India | 24 | 58 | Inquiry |
| Valens Molecules | +91-040-23417926 +91-4023417926 | Hyderabad, India | 10 | 58 | Inquiry |
| Ralington Pharma | +91-7948911722 +91-9687771722 | Gujarat, India | 1350 | 58 | Inquiry |
| Mehta Medicare Pvt., Ltd. | 91-33-24767882 | Kolkata, India | 61 | 58 | Inquiry |
| SynZeal Research Pvt Ltd | +1 226-802-2078 | Gujarat, India | 6522 | 58 | Inquiry |
| VALENS MOLECULES PRIVATE LTD | + 91 40 2341 8086 / 7926 | New Delhi, India | 11 | 58 | Inquiry |
| Indogulf Group | 91-22-23455220 | Maharashtra, India | 249 | 58 | Inquiry |
| Ralington Pharma | 91-79-48911722 | Gujarat, India | 312 | 58 | Inquiry |
| Abil Chempharma Pvt. Ltd. | 91-22-62599828 | Maharashtra, India | 38 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| GLP Pharma Standards | 58 |
| Virchow Groups | 58 |
| Valens Molecules | 58 |
| Ralington Pharma | 58 |
| Mehta Medicare Pvt., Ltd. | 58 |
| SynZeal Research Pvt Ltd | 58 |
| VALENS MOLECULES PRIVATE LTD | 58 |
| Indogulf Group | 58 |
| Ralington Pharma | 58 |
| Abil Chempharma Pvt. Ltd. | 58 |
599-79-1(Sulfasalazine)Related Search:
1of4
chevron_right




