Budesonide
- CAS No.
- 51333-22-3
- Chemical Name:
- Budesonide
- Synonyms
- PULMICORT;Budeson;Respules;RHINOCORT;Budenofalk;Budesonide CRS;BIDIEN;S-1320;Entocort;Micronyl
- CBNumber:
- CB0313122
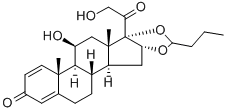
- Molecular Formula:
- C25H34O6
- Molecular Weight:
- 430.53
- MOL File:
- 51333-22-3.mol
- Modify Date:
- 2024/4/3 17:07:09
| Melting point | 221-232°C (dec.) |
|---|---|
| Boiling point | 464.79°C (rough estimate) |
| alpha | D25 +98.9° (c = 0.28 in methylene chloride) |
| Density | 1.1046 (rough estimate) |
| refractive index | 1.4593 (estimate) |
| storage temp. | Store at RT |
| solubility | Practically insoluble in water, freely soluble in methylene chloride, sparingly soluble in ethanol (96 per cent). |
| form | powder |
| pka | 12.87±0.10(Predicted) |
| color | White to Off-White |
| Water Solubility | 21.53mg/L(temperature not stated) |
| Merck | 14,1468 |
| BCS Class | 2 |
| InChIKey | VOVIALXJUBGFJZ-KWVAZRHASA-N |
| CAS DataBase Reference | 51333-22-3(CAS DataBase Reference) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS08,GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H361-H332-H317-H334-H312 | |||||||||
| Precautionary statements | P261-P272-P280-P302+P352-P333+P313-P321-P363-P501-P201-P202-P281-P308+P313-P405-P501-P261-P271-P304+P340-P312-P280-P302+P352-P312-P322-P363-P501-P261-P285-P304+P341-P342+P311-P501 | |||||||||
| Hazard Codes | Xn | |||||||||
| Risk Statements | 40-36/37/38-20/21/22 | |||||||||
| Safety Statements | 22-36-26 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | TU3723000 | |||||||||
| HS Code | 29372900 | |||||||||
| Toxicity | LD50 oral in rat: > 3200mg/kg | |||||||||
| NFPA 704 |
|
Budesonide price More Price(2)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | PHR1178 | Budesonide Pharmaceutical Secondary Standard; Certified Reference Material | 51333-22-3 | 500MG | ₹10327.05 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | B7777 | Budesonide ≥99% | 51333-22-3 | 250MG | ₹27333.13 | 2022-06-14 | Buy |
Budesonide Chemical Properties,Uses,Production
Description
Budesonide is composed of a 1:1 mixture of epimers of the 16,17-butylacetal, creating a chiral center. The 22R-epimer binds to the GR with higher affinity than does the 22S-epimer (Table 33.5). The butyl acetal chain provided the highest potency for the homologous acetal chains. Its rate of topical uptake into epithelial tissue is more than 100 times faster than that for hydrocortisone and dexamethasone. Approximately 85% of the IV administered dose of budesonide undergoes extensive first-pass hepatic metabolism by CYP3A4 to its primary metabolites, 6β-hydroxybudesonide and 16α-hydroxyprednisolone, which have approximately 1/100 the potency of budesonide. This is an important inactivation step in limiting budesonide's systemic effect on adrenal suppression.
Chemical Properties
White Solid
Uses
Budesonide is a glucocorticoid steroid that activates the glucorcorticoid receptor with an EC50 value of 12.4 nM. Like other glucocorticoids, budesonide reduces inflammation and has utility in inflammatory diseases, like asthma and inflammatory bowel disease. Also like other glucocorticoids, budesonide may be abused by athletes.[Cayman Chemical]
Definition
ChEBI: A glucocorticoid steroid having a highly oxygenated pregna-1,4-diene structure. It is used mainly in the treatment of asthma and non-infectious rhinitis and for treatment and prevention of nasal polyposis.
Indications
Budesonide is a synthetic corticosteroid having a potent glucocorticoid and weak mineralocorticoid activity. In standard in vitro and animal models, budesonide has an approximately 200-fold higher affinity for the glucocorticoid receptor and a 1000-fold higher topical antiinflammatory potency than cortisol.
General Description
Budesonide (Pulmicort Turbohaler,Rhinocort) is extensively metabolized in the liver, with 85%to 95% of the orally absorbed drug metabolized by the firstpasseffect. The major metabolites are 6β-hydroxybudesonideand 16α-hydroxyprednisolone, both with less than1% of the activity of the parent compound. Metabolism involvesthe CYP3A4 enzyme, so coadministration of budesonidewith a known CYP3A4 inhibitor should be monitoredcarefully.
Biological Activity
Synthetic anti-inflammatory glucocorticoid that displays chemopreventive activity. Prevents formation of lung adenomas and adenocarcinomas in mice following inhalation or oral administration. Reverses DNA hypomethylation and modulates expression of cancer related genes.
Mechanism of action
Budesonide is an acetal formed between the 16α,17α-dihydroxyl groups and butanal. It is a nonhalogenated glucocorticoid with a 16,17-acetal that decreases the mineralocorticoid activity. In receptor affinity studies, the R-epimer was twofold more active than the S-epimer. Because the C-21 hydroxy is free, budesonide is not a prodrug and is active as administered. Only 34% of the metered dose of inhaled budesonide reaches the lung.
Pharmacology
While budesonide is well absorbed from the GI tract, its oral bioavailability is low (about 10%), primarily because of extensive first-pass metabolism in the liver. Two major metabolites (16α-hydroxyprednisolone and 6β- hydroxybudesonide) are formed via the cytochrome P450 3A enzyme. In vitro studies on the binding of the two primary metabolites to the corticosteroid receptor indicate that their affinity for the receptor is less than 1% of that of the parent compound. It is hoped that use of this drug will avoid the long-term adverse reactions seen with systemically active corticosteroids.
Clinical Use
Recently, budesonide (Entecort EC) has been approved for the treatment of mildly to moderately active Crohn’s disease involving the ileum and/or ascending colon.
Metabolism
Budesonide was metabolized three- to sixfold more rapidly than triamcinolone acetonide. The pharmacokinetics of budesonide after inhalation, oral, and IV administration displayed a mean plasma half-life of 2.8 hours and a systemic bioavailability of approximately 10% after oral administration (Table 33.5) (101). Pulmonary bioavailability is less than 40% after inhalation (70–75% after correction for the amounts of budesonide deposited in the inhalation device and oral cavity). No oxidative metabolism was observed in the lung. When given by inhalation, 32% of the dose is excreted in the urine as metabolites, 15% in the feces, and 41% of the dose remained in the mouthpiece of the inhaler. Following intranasal administration, very little of intranasal budesonide is absorbed from the nasal mucosa. Much of the intranasal dose (~60%) was swallowed, however, and remained in the GI tract to be excreted unchanged in the feces, whereas that fraction of the intranasal dose that was absorbed was extensively metabolized.
Budesonide Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| AARTI INDUSTRIES LIMITED | 912267976666 | New Delhi, India | 172 | 58 | Inquiry |
| Jigs Chemical ltd | +919099003427 | Gujarat, India | 239 | 58 | Inquiry |
| Halcyon Labs Pvt. Ltd | +919833750169 | Mumbai, India | 17 | 58 | Inquiry |
| Micro Orgo Chem | +91-91-022-24969510 +91-9082984052 | Mumbai, India | 66 | 58 | Inquiry |
| Anant Pharmaceuticals Pvt Ltd | +91-8550986868 +91-9485998001 | Haryana, India | 461 | 58 | Inquiry |
| Coral Drugs Pvt Ltd | +91-1244518100 +91-8199986118 | New Delhi, India | 24 | 58 | Inquiry |
| GLP Pharma Standards | +91 9866074638 | Hyderabad, India | 1644 | 58 | Inquiry |
| Gonane Pharma | +91-9819380043 +91-9819380043 | NaviMumbai, India | 192 | 58 | Inquiry |
| Apotex Pharmachem India Pvt Ltd | +91-8022891034 +91-8022891000 | Karnataka, India | 109 | 58 | Inquiry |
| Vamsi Labs Ltd | +91-2172357274 +91-9891966300 | Maharashtra, India | 43 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| AARTI INDUSTRIES LIMITED | 58 |
| Jigs Chemical ltd | 58 |
| Halcyon Labs Pvt. Ltd | 58 |
| Micro Orgo Chem | 58 |
| Anant Pharmaceuticals Pvt Ltd | 58 |
| Coral Drugs Pvt Ltd | 58 |
| GLP Pharma Standards | 58 |
| Gonane Pharma | 58 |
| Apotex Pharmachem India Pvt Ltd | 58 |
| Vamsi Labs Ltd | 58 |
Related articles
- Budesonide: Uses, Mechenism of action, and Side effects
- Budesonide, sold under the brand name Pulmicort, among others, is a corticosteroid medication.
- Mar 22,2024
51333-22-3(Budesonide)Related Search:
1of4
chevron_right




