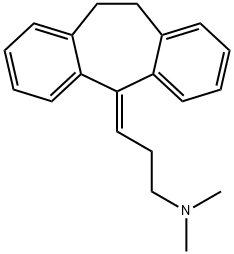
Amitriptyline
- CAS No.
- 50-48-6
- Chemical Name:
- Amitriptyline
- Synonyms
- Amitriptylin;amitriptilina;Amitryptiline;Amitryptyline;Elavil;5-(3-dimethylaminopropylidene)-10,11-dihyro-5h-dibenzo(a,d)cycloheptene;5-(3-Dimethylaminopropylidene)-10,11-dihydro-5H-dibenzo(a,d)cycloheptatriene;n750;elani;mk230
- CBNumber:
- CB1159271
- Molecular Formula:
- C20H23N
- Molecular Weight:
- 277.4
- MOL File:
- 50-48-6.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/12/18 14:07:02
| Melting point | 196-197°C |
|---|---|
| Boiling point | 410.26°C (rough estimate) |
| Density | 0.9415 (rough estimate) |
| refractive index | 1.7500 (estimate) |
| storage temp. | Store at -20°C |
| form | Liquid |
| pka | 9.4(at 25℃) |
| color | Colorless to light yellow |
| Water Solubility | 9.7 mg/mL |
| Stability | Light Sensitive |
| CAS DataBase Reference | 50-48-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Amitriptyline(50-48-6) |
| EPA Substance Registry System | Amitriptyline (50-48-6) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS08,GHS09,GHS05 |
|---|---|
| Signal word | Danger |
| Hazard statements | H361-H301+H311+H331-H410-H318 |
| Precautionary statements | P273-P391-P501-P201-P202-P281-P308+P313-P405-P501-P280-P305+P351+P338-P310 |
| HazardClass | IRRITANT |
| Hazardous Substances Data | 50-48-6(Hazardous Substances Data) |
Amitriptyline Chemical Properties,Uses,Production
Uses
Amitriptyline is used for anxious-depressive conditions. It is easier to tolerate than imipramine.
Definition
ChEBI: An organic tricyclic compound that is 10,11-dihydro-5H-dibenzo[a,d][7]annulene substituted by a 3-(dimethylamino)propylidene group at position 5.
World Health Organization (WHO)
Amitriptyline, a tricyclic antidepressant was introduced in 1961 for the management of endogenous depression and is listed in the 8th WHO Model List of Essential Drugs. Much of the adverse effects are caused by its antimuscarinic actions. These include dry mouth, cardiac arrhythmias, central nervous system disturbances, blood disorders and risk of suicide. The risk of suicide and dangers related to overdosage led the Norwegian Medicines Control Authority to put the higher strength formulation under prescribing restriction in 1992. The risk of death following overdosage is apparently higher for products containing tricyclic compounds as compared with nontricyclic products.
Biological Functions
Amitriptyline is a tertiary amine dibenzocycloheptadiene TCA with a propylidene side chain extending from the central carbocyclic ring. The diarylpropylideneamine moiety for amitriptyline makes it sensitive to photo-oxidation; therefore, its hydrochloride solutions should be protected from light to avoid ketone formation and precipitation.
Pharmacokinetics
Amitriptyline is rapidly absorbed from the GI tract and from parenteral sites.Amitriptyline and its active metabolite, nortriptyline, are distributed into breast milk. Amitriptyline is primarily (65%) metabolized by N-demethylation by CYP2D6 to nortriptyline and hydroxylation to its E-10-hydroxy metabolite. Nortriptyline is pharmacologically active as a secondary amine TCA. Amitriptyline shows approximately equal affinity for 5-HT and NE transporters.
Amitriptyline Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Anant Pharmaceuticals Pvt Ltd | +91-8550986868 +91-9485998001 | Haryana, India | 460 | 58 | Inquiry |
| KPS Chemicals And Pharmaceuticals | +91-9870076629 +91-8469484608 | Gujarat, India | 33 | 58 | Inquiry |
| Humble Healthcare Limited | +91-9720093000 +91-8006400378 | Uttar Pradesh, India | 140 | 58 | Inquiry |
| AKASH PHARMA EXPORTS | +91-9388123451 +91-9846039283 | Kerela, India | 470 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6739 | 58 | Inquiry |
| Astitva Chemicals | 08048262675 | Gujarat, India | 89 | 58 | Inquiry |
| SynZeal Research Pvt Ltd | +1 226-802-2078 | Gujarat, India | 6514 | 58 | Inquiry |
| Pharma Affiliates | 172-5066494 | Haryana, India | 6754 | 58 | Inquiry |
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 | China | 2930 | 58 | Inquiry |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | China | 49732 | 58 | Inquiry |
50-48-6(Amitriptyline)Related Search:
1of4
chevron_right




