Silica glass
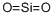
- CAS No.
- 60676-86-0
- Chemical Name:
- Silica glass
- Synonyms
- KIESELGUHR;SILICONE DIOXIDE;CELITE 545;HYFLO;MICROSILICA;fused;Fused silica;KIESELGEL;KIESELGUR;FILTER AGENT
- CBNumber:
- CB1199394
- Molecular Formula:
- O2Si
- Molecular Weight:
- 60.0843
- MOL File:
- 60676-86-0.mol
- Modify Date:
- 2024/1/9 17:36:53
| Melting point | 1610 °C(lit.) |
|---|---|
| Boiling point | 2950°C |
| Density | 2.6 g/mL at 25 °C(lit.) |
| refractive index |
n |
| solubility | insoluble in H2O, acid solutions; soluble in HF |
| form | rod (1/8") |
| CAS DataBase Reference | 60676-86-0 |
| NIST Chemistry Reference | Silicon dioxide(60676-86-0) |
| EPA Substance Registry System | Silica vitreous (60676-86-0) |
| Modulus of Elasticity | 70.0 - 78.0 GPa |
|---|---|
| Poissons Ratio | 0.17 |
| Shear Modulus | 31.0 GPa |
| Hardness, Mohs | 5.5 - 7.0 |
| Hardness, Knoop | 820 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS08 |
|---|---|
| Signal word | Warning |
| Hazard statements | H319-H335-H373 |
| Precautionary statements | P305+P351+P338-P314 |
| Risk Statements | 48/20-36/38-45-36/37 |
| Safety Statements | 22-24/25-45-26-53-36 |
| OEB | D |
| OEL | TWA: 0.05 mg/m3 |
| WGK Germany | 2 |
| RTECS | VV7311000 |
| HS Code | 28112200 |
| Toxicity | LDLo intravenous in cat: 5mg/kg |
Silica glass price More Price(21)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 914150 | Silica dioxide- sorbent milled nanofiber | 60676-86-0 | 5G | ₹19041.18 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 914150 | Silica dioxide- sorbent milled nanofiber | 60676-86-0 | 10G | ₹33243.58 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 912999 | Silica dioxide- vs nanofiber | 60676-86-0 | 5G | ₹8454.33 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 912999 | Silica dioxide- vs nanofiber | 60676-86-0 | 10G | ₹14819.43 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 912492 | Silica dioxide- sorbent nanofiber | 60676-86-0 | 5G | ₹16865.35 | 2022-06-14 | Buy |
Silica glass Chemical Properties,Uses,Production
Chemical Properties
Made up of spherical submicroscopic particles under 0.1 micron in size.
Occurrence
This material is known largely as a synthetic material, but there are instances of the material occurring in nature. Vitreous tubes called fulgurites are produced when lightning fuses quartz sand. Large deposits of fulgurite exist in the Libyan desert. Vitreous silica can also be produced by meteor impact. The impact leads to rapid adiabatic heating of the quartz above its melting point. The quartz forms a glass on cooling. Examples of this type of vitreous silica have been found near Canyon Diablo, Arizona, and in meteorite craters in Australia and Arabia.
Uses
Concrete, grouts, mortars, elastomers, refrac- tory and coating applications.
Production Methods
Modern manufacturing processes of vitreous typically involve the fusion or viscous sintering of silica particles; the particles can be derived from sand crystals or are produced through a chemical process, e.g., flame hydrolysis or sol–gel. In one practice of the flame hydrolysis process, the powder is produced and fused into glass a single step, without the isolation of a porous body. Dopant and additive profiles are concentration are then controlled by the deposition conditions. When a process involving a discrete porous silica body as an intermediate is used, subsequent processing steps can be used to control dopant levels and in particular, the hydroxyl level of the final glass. The choice of fabrication method is often dictated by the end-use specifications. Flame hydrolysis or similar chemical techniques that allow for the production of very high purity glass are the methods of choice for optical applications but may be economically wasteful for less demanding applications.
Translucent Vitreous Silica. Translucent vitreous silica is produced by fusion of high purity quartz sand crystals. Sand is packed around a graphite rod through which a current is passed. The resistance heating produces a plastic mass that can be blown into molds, drawn into tubing, or shaped by rolling or pressing. Separation from the graphite rod is facilitated by gaseous products formed by interfacial reaction. Because the outside is sandy, the product is known as sand-surface ware. A matte finish is obtained by mechanical buffing. A glazed surface is produced by fusing the outside surface with an electric carbon arc or flame.
Transparent Vitreous Silica. Clear, transparent, bubble-free vitreous silica may be obtained by melting natural quartz minerals by flame or plasma vapor deposition (synthetic fused silicas), and by sol–gel processing.
General Description
Silicon dioxide is one of the important constituents of sedimentary rock bauxite, basalt fibers and ceramic fibers. It is added to cement for improving the hydraulic properties of cement.
Hazard
Questionable carcinogen.
Safety Profile
An inhalation hazard. Questionable carcinogen with experimental tumorigenic data. Poison by intraperitoneal, intravenous, and intratracheal routes. See also other shca entries.
Potential Exposure
Amorphous fumed silica is used as a mineral, natural or synthetic fiber. A potential danger to those involved in the production and handling of fumed silica for paint pigments or catalysts. Diatomaceous earth is used in clarifying liquids, in manufacture of fire brick and heat insulators; used as a filtering agent; as a filler in construction materials; pesticides, paints, and varnishes. A potential danger to those involved in mining of diatomaceous earth or fabrication of products there from.
Purification Methods
Purification of silica for high technology applications uses isopiestic vapour distillation from concentrated volatile acids and is absorbed in high purity water. The impurities remain behind. Preliminary cleaning to remove surface contaminants uses dip etching in HF or a mixture of HCl, H2O2 and deionised water [Phelan & Powell Analyst 109 1299 1984].
Incompatibilities
Silica, amorphous is a noncombustible solid. Generally unreactive chemically. Incompatible with fluorine, oxygen difluoride, chlorine trifluoride. Soluble in molten alkalis and reacts with most metallic oxides at high temperature.
Waste Disposal
Sanitary landfill.
Silica glass Preparation Products And Raw materials
Raw materials
Preparation Products
1of2
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| ARRAKIS INDUSTRIES LLP | +91 74995 32711 | Maharashtra, India | 1353 | 58 | Inquiry |
| Biokemix | +91 (40) 6662-6366 | New Delhi, India | 486 | 50 | Inquiry |
| CHEMSWORTH | +91-261-2397244 | New Delhi, India | 6700 | 30 | Inquiry |
| Otto Chemie Pvt. Ltd. | +91 9820041841 | Mumbai, India | 5870 | 58 | Inquiry |
| Central Drug House(P) Ltd. | 91-11-49404040 | New Delhi, India | 6157 | 58 | Inquiry |
| Aritech Chemazone Private Limited | +91-9034345475 | Punjab, India | 684 | 58 | Inquiry |
| Triveni chemicals | 08048762458 | New Delhi, India | 6088 | 58 | Inquiry |
| LOBA CHEMIE PVT.LTD. | 91-22-6663 6699 | Mumbai, India | 3075 | 58 | Inquiry |
| Shilpa Enterprises | 08048963201 | Nagpur, India | 3 | 58 | Inquiry |
| Techinstro | 08047631862 | Nagpur, India | 9 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| ARRAKIS INDUSTRIES LLP | 58 |
| Biokemix | 50 |
| CHEMSWORTH | 30 |
| Otto Chemie Pvt. Ltd. | 58 |
| Central Drug House(P) Ltd. | 58 |
| Aritech Chemazone Private Limited | 58 |
| Triveni chemicals | 58 |
| LOBA CHEMIE PVT.LTD. | 58 |
| Shilpa Enterprises | 58 |
| Techinstro | 58 |
Related articles
- Vitreous or Amorphous Silica
- High-purity amorphous or fused silica, also called vitreous silica, when optically translucent is a high-performance ceramic o....
- Jan 9,2024
60676-86-0(Silica glass)Related Search:
1of5
chevron_right




