Lopinavir
- CAS No.
- 192725-17-0
- Chemical Name:
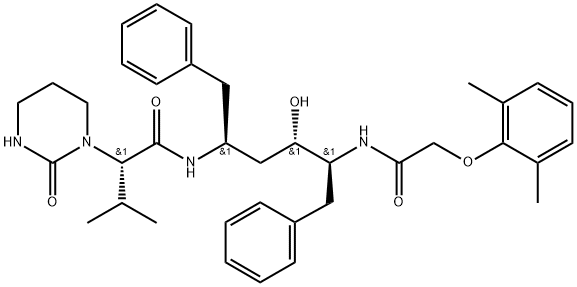
- Lopinavir
- Synonyms
- Lopinavir CRS;(2S)-N-[(2S,4S,5S)-5-[2-(2,6-diMethylphenoxy)acetaMido]-4-hydroxy-1,6-diphenylhexan-2-yl]-3-Methyl-2-(2-oxo-1,3-diazinan-1-yl)butanaMide;(2s)-n-[(2r,4s,5s)-5-[[2-(2,6-dimethylphenoxy)acetyl]amino]-4-hydroxy-1,6-diphenyl-hexan-2-yl]-3-methyl-2-(2-oxo-1,3-diazinan-1-yl)butanamide;(aS)-N-[(1S,3S,4S)-4-[[2-(2,6-Dimethylphenoxy)acetyl]amino]-3-hydroxy-5-phenyl-1-(phenylmethyl)pentyl]tetrahydro-a-(1-methylethyl)-2-oxo-1(2H)-Pyrimidineacetamide;Koletr;134368;CS-546;Koletra;ABT 378;Aluvia)
- CBNumber:
- CB3663640
- Molecular Formula:
- C37H48N4O5
- Molecular Weight:
- 628.81
- MOL File:
- 192725-17-0.mol
- Modify Date:
- 2023/6/30 15:45:59
| Melting point | 255.2-260.6 °F (124—127°C) |
|---|---|
| Boiling point | 924.1±65.0 °C(Predicted) |
| Density | 1.163±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMSO: soluble20mg/mL, clear |
| pka | 13.89±0.46(Predicted) |
| form | powder |
| color | white to beige |
| optical activity | [α]/D -20 to -27°, c = 0.4 in methanol |
| Stability | Hygroscopic |
| CAS DataBase Reference | 192725-17-0(CAS DataBase Reference) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H373-H319-H335 | |||||||||
| Precautionary statements | P264-P280-P302+P352-P321-P332+P313-P362-P264-P280-P305+P351+P338-P337+P313P-P260-P314-P501 | |||||||||
| RIDADR | 3077 | |||||||||
| HS Code | 29335990 | |||||||||
| NFPA 704 |
|
Lopinavir price More Price(3)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | SML1222 | Lopinavir ≥98% (HPLC) | 192725-17-0 | 10MG | ₹2686.2 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | SML1222 | Lopinavir ≥98% (HPLC) | 192725-17-0 | 50MG | ₹6882 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | PHR1927 | Lopinavir Pharmaceutical Secondary Standard; Certified Reference Material | 192725-17-0 | 1G | ₹29584.73 | 2022-06-14 | Buy |
Lopinavir Chemical Properties,Uses,Production
Description
Lopinavir, the sixth HIV protease inhibitor in the “navir” class, was launched in coformulation with ritonavir, another HIV protease inhibitor already marketed (Abbott, 1996); this original formulation was introduced as Kaletra for use in combination with either nucleoside or non-nucleoside reverse transcriptase inhibitors for the treatment of AIDS in adults and children. Lopinavir is a peptidomimetic compound with a structural core identical to that of ritonavir, on which terminal groups, particularly a modified valine, were introduced by peptide coupling procedures. Lopinavir is a potent competitive inhibitor of HIV-I protease exhibiting high potential against ritonavir-resistant mutations. In several animal species, pharmacokinetic studies with the lopinavirlritonavir association showed that the modest properties of lopinavir were significantly improved in presence of ritonavir, in terms of Cmax and duration of action. Ritonavir inhibits the P450 isoenzyme CYP3A4 and the human liver microsomal metabolism of lopinavir, so strongly amplifying plasma levels of this latter component. In AIDS patients, the plasma HIV RNA level was considerably reduced and the CD4+ T-cell counts increased after administration of lopinavir combined with relatively small doses of ritonavir. Kaletra is intended to be used jointly with other antiretroviral agents.
Chemical Properties
Lopinavir is a white to light tan powder. It is freely soluble in methanol and ethanol, soluble in isopropanol and practically insoluble in water.
Uses
Lopinavir is a potent HIV protease inhibitor with Ki of 1.3 pM
Indications
Lopinavir is available in the United States only as a fixed-dose combination with ritonavir (Kaletra). In this regimen, a low dose of ritonavir is used to inhibit the rapid inactivation of lopinavir by CYP3A4.
Definition
ChEBI: Lopinavir is a dicarboxylic acid diamide that is amphetamine is substituted on nitrogen by a (2,6-dimethylphenoxy)acetyl group and on the carbon alpha- to nitrogen by a (1S,3S)-1-hydroxy-3-{[(2S)-3-methyl-2-(2-oxotetrahydropyrimidin-1-yl)butanoyl]amino}-4-phenylbutyl group. An antiretroviral of the protease inhibitor class, it is used against HIV infections as a fixed-dose combination with another protease inhibitor, ritonavir. It has a role as an antiviral drug, a HIV protease inhibitor and an anticoronaviral agent. It is a member of amphetamines and a dicarboxylic acid diamide.
Antimicrobial activity
Lopinavir is active against HIV-1 and HIV-2.
Acquired resistance
Significant resistance to the antiretroviral efficacy of ritonavirbooted lopinavir occurs as a result of amino acid substitutions at positions 32, 47 and 82 in the protease region. Protease inhibitor resistance is uncommon in patients identified with early failure of combination therapy with ritonavir boostedlopinavir and nucleotide reverse transcriptase inhibitors.
General Description
Lopinavir is a protease inhibitor that has been approved foruse in combination with ritonavir for patients with HIV whohave not responded to other treatment modalities. Lopinaviris used in excess over ritonavir. Ritonavir at amounts givenhas no antiretroviral activity, Ritonavir inhibits lopinavir’smetabolism by CYP3A4, causing a higher level of lopinavirin the system. The combination is the first protease inhibitorapproved for patients as young as 6 months of age.
Pharmacokinetics
Oral absorption: Not known/available
Cmax 400 mg + ritonavir 100 mg twice daily: c. 9.6 mg/L
Cmin 400 mg + ritonavir 100 mg twice daily: c. 5.5 mg/L
Plasma half-life: c. 5–6 h
Volume of distribution: Not known/available
Plasma protein binding: c. 98–99%
Absorption and distribution
The absorption of lopinavir–ritonavir in capsule or liquid form is favorably affected by the presence of food, particularly if high in fat. The CNS penetration is good. It has a semen:plasma ratio of 0.07. It is distributed into breast milk.
Metabolism
Lopinavir is extensively metabolized by the CYP3A4 system, but this is inhibited by ritonavir.
Excretion
Over an 8-day period after single dosing with the combined formulation, around 10% and 83% of the administered dose is recovered in urine and feces, respectively. Less than 3% of the dose is recovered as unchanged drug in urine and 20% in feces. In mild to moderate hepatic impairment, an increase in exposure of approximately 30% is observed, but is probably not clinically relevant. It should be avoided in severe hepatic impairment.
Clinical Use
Treatment of HIV infection (in combination with ritonavir and other antiretroviral agents)
Side effects
The most common adverse events seen in trials of complex antiretroviral regimens were diarrhea, nausea, headache, fatigue, vomiting and rash. Ritonavir-boosted lopinavir is associated with a dyslipidemia profile characteristic of those treated with other protease inhibitors boosted with 200 mg of ritonavir.
Lopinavir Preparation Products And Raw materials
Raw materials
1of3
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| J S LABS | +91-7330612784 +91-7330612784 | Tamil Nadu, India | 160 | 58 | Inquiry |
| SEUTIC | +91-8309787199 +91-8309787199 | Hyderabad, India | 124 | 58 | Inquiry |
| SUJALAM CHEMICALS | +91-9422560788 +91-9422560788 | Maharashtra, India | 42 | 58 | Inquiry |
| TYNDAL LABS PVT LTD | +91-8008166674 +91-8008166674 | AndhraPradesh, India | 95 | 58 | Inquiry |
| Hetero Drugs Limited | +91-4023704923 +91-4023704923 | Telangana, India | 296 | 58 | Inquiry |
| Venture Pharmaceuticals pvt ltd | +91-9173909075 +91-9173909075 | Gujarat, India | 45 | 58 | Inquiry |
| HRV Global Life Sciences | +91-9820219686 +91-9820219686 | Telangana, India | 379 | 58 | Inquiry |
| Aurobindo Pharma Limited | +914066725000 | Telangana, India | 112 | 58 | Inquiry |
| Cipla Ltd | +912224826000 | Maharashtra, India | 133 | 58 | Inquiry |
| Amara Labs Pvt Ltd | +91-9553279972 +91-9100091565 | Hyderabad, India | 42 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| J S LABS | 58 |
| SEUTIC | 58 |
| SUJALAM CHEMICALS | 58 |
| TYNDAL LABS PVT LTD | 58 |
| Hetero Drugs Limited | 58 |
| Venture Pharmaceuticals pvt ltd | 58 |
| HRV Global Life Sciences | 58 |
| Aurobindo Pharma Limited | 58 |
| Cipla Ltd | 58 |
| Amara Labs Pvt Ltd | 58 |
Related articles
- Side effects of Lopinavir
- Lopinavir (formerly known as ABT-378) is an HIV-1-specific protease inhibitor engineered specifically to address the shortcomi....
- Apr 1,2022
192725-17-0(Lopinavir)Related Search:
1of4
chevron_right




