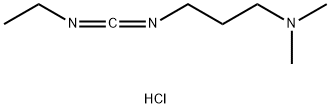
1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride
- CAS No.
- 25952-53-8
- Chemical Name:
- 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride
- Synonyms
- EDCI;EDC.HCl;EDAC;EDARAVONE;WSC;1-ETHYL-3-(3-DIMETHYLAMINOPROPYL) CARBODIIMIDE;WSCI;WSCD;1-Ethyl-3-(3-dimethyllaminopropyl)carbodiimide hydrochloride;EDC.HCI
- CBNumber:
- CB7403031
- Molecular Formula:
- C8H18ClN3
- Molecular Weight:
- 191.7
- MOL File:
- 25952-53-8.mol
- Modify Date:
- 2024/8/28 16:27:48
| Melting point | 110-115 °C(lit.) |
|---|---|
| Density | 0.877 g/mL at 20 °C(lit.) |
| vapor pressure | 0.002Pa at 20℃ |
| refractive index |
n |
| storage temp. | -20°C |
| solubility | H2O: soluble1 gm/10 ml, clear to very slightly hazy, colorless to very faintly yellow |
| form | Crystalline Powder |
| color | White to off-white |
| Water Solubility | Soluble |
| Sensitive | Hygroscopic |
| BRN | 5764110 |
| Stability | Stable, but sensitive to moisture. Incompatible with strong acids, strong oxidizing agents, moisture. |
| InChIKey | FPQQSJJWHUJYPU-UHFFFAOYSA-N |
| LogP | -2.98 at 25℃ |
| CAS DataBase Reference | 25952-53-8(CAS DataBase Reference) |
| EPA Substance Registry System | 1,3-Propanediamine, N'-(ethylcarbonimidoyl)-N,N-dimethyl-, monohydrochloride (25952-53-8) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS06,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H302-H311-H315-H317-H373-H410 | |||||||||
| Precautionary statements | P260-P273-P280-P301+P312-P302+P352+P312-P314 | |||||||||
| Hazard Codes | C,Xi | |||||||||
| Risk Statements | 34-36/37/38-41-37/38-20/21/22 | |||||||||
| Safety Statements | 26-36/37/39-45-37/39-36 | |||||||||
| RIDADR | UN 2735 8/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | FF2200000 | |||||||||
| F | 1-3-10 | |||||||||
| Hazard Note | Irritant | |||||||||
| TSCA | Y | |||||||||
| HS Code | 29252000 | |||||||||
| Toxicity | LD50 intravenous in mouse: 56mg/kg | |||||||||
| NFPA 704 |
|
1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride price More Price(47)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | E7750 | N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride commercial grade, powder | 25952-53-8 | 100MG | ₹2392.33 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | E7750 | N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride commercial grade, powder | 25952-53-8 | 1G | ₹3366.58 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | E7750 | N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride commercial grade, powder | 25952-53-8 | 5G | ₹5076.93 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | E7750 | N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride commercial grade, powder | 25952-53-8 | 10G | ₹9396.1 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | E7750 | N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride commercial grade, powder | 25952-53-8 | 25G | ₹19300.98 | 2022-06-14 | Buy |
1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride Chemical Properties,Uses,Production
Chemical Properties
EDC-HCl, or 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, is a white crystalline powder that easily deliquescent and can dissolve in both water and ethanol. It is commonly used as an activating reagent in amide synthesis to activate carboxyl groups. It can also activate phosphate groups, cross-link proteins and nucleic acids, and produce immunocouplers. EDC-HCl is commonly applied in the pH range of 4.0-6.0 and is often used in conjunction with N-Hydroxysuccinimide (NHS) or N-Hydroxysulfosuccinimide sodium salt to improve coupling efficiency.
Uses
1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride is used as a carboxyl activating agent. It plays a vital role for immobilization of large biomolecules in association with N-hydroxysuccinimide. It is also used in the acylation of phosphoranes.
Application
1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride is water-soluble carbodiimide, widely used for peptide coupling.
N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride has been used for the formation of FND (fluorescent nanodiamonds)-transferrin bioconjugates.
It has been used for crosslinking polyethylenimine to gold particles.
It has been used as a carbodiimide linkage agent for coating of carboxylated polystyrene beads with biotinylated BSA (bovine serum albumin).
General Description
EDC hydrochloride is a water-soluble derivative of carbodiimide useful for conjugating haptens to proteins and polypeptides. Used to modify NMDA receptors and as a condensing agent in peptide synthesis. The major advantage of EDAC coupling is the easy removal of excess reagent and the corresponding urea by washing with dilute acid or water. Carbodiimides catalyze the formation of amide bonds, carboxylic acids, and amines by activating the carboxylate to form an O-acylurea. This intermediate can be attacked by an amine directly to form an amide. EDAC is released as a soluble urea derivative.
Biochem/physiol Actions
1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride(25952-53-8) is a water soluble condensing reagent. EDAC is generally utilized as a carboxyl activating agent for amide bonding with primary amines. Additionally, it reacts with phosphate groups. It has been utilized in peptide synthesis, crosslinking proteins to nucleic acids as well as preparation of immunoconjugates.
Mechanism of action
Carboxyl group activation with EDC. xHCl proceeds similarly to other carbodiimide couplings. The reaction of a carboxylic acid and the carbodiimide forms an O-acylisourea intermediate. The O-acylisourea is a highly reactive species that readily reacts with amines, peptide coupling additives, or reducing agents. EDC. HCl is transformed into the corresponding urea during coupling reactions. It has the advantage over DCU in that it can be removed from the reaction mixture by extraction or be washed out from solid phase synthesis applications. However, the O-acylisourea can rearrange irreversibly to an N-acyl urea and racemise the α-carbon of the amino acid via the formation of an oxazol-4(5H)-one [azlactone]. N-Acylurea formation and racemisation may be reduced by using intermediate nucleophiles, which convert the O-acylurea to an activated ester containing the nucleophile. The mechanism of amide bond formation applies both to EDC and EDC.HCl. The differences are that the free base can be applied as a bifunctional reagent: a coupling agent (carbodiimide) and a base (dimethylamino). At the same time, the HCl salt can form an O-acylisourea cyclic intermediate.
Synthesis
1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride can be used for the synthesis of amides.It is used as a coupling agent in the synthesis of esters from carboxylic acids using dimethylaminopyridine as the catalyst.
Preparation method of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride(CN104193654A)
Purification Methods
It is an excellent H2O-soluble peptide coupling reagent. It is purified by dissolving (ca 1g) in CH2Cl2 (10mL) at room temperature and then add dry Et2O (~110mL) dropwise and the crystals that separate are collected, washed with dry Et2O, recrystallised from CH2Cl2/Et2O and dried in a vacuum over P2O5. It is important to work in a dry atmosphere or work rapidly and then dry the solid as soon as possible. The material is moderately hygroscopic, but once it becomes wet it reacts slowly with H2O. Store it away from moisture at -20o to slow down the hydrolysis process. The free base has b 47-48o/0.27mm, 53-54o/0.6mm, n 1.4582. The methiodide is recrystallised from CHCl3/EtOAc, the crystals are filtered off, washed with dry Et2O, recrystallised from CHCl3/Et2O, and dried in vacuo over P2O5, m 93-95o, 94-95o. [Sheehan et al. J Am Chem Soc 87 2492 1965, Sheehan & Cruickshank Org Synth Coll Vol V 555 1973.] § A polymer bound version is commercially available.
1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| BTC pharm India | +918790379245 | AndhraPradesh, India | 1269 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Mumbai, India | 66 | 58 | Inquiry |
| SAGAR LIFE SCIENCES PVT LTD | +91-8770359041 +91-8770359041 | Gujarat, India | 79 | 58 | Inquiry |
| ANJI BIOSCIENCES | +91-9000100077 +91-9000100077 | Hyderabad, India | 430 | 58 | Inquiry |
| LIFECHEM PHARMA | +91-9825440401 +91-9825440401 | Gujarat, India | 25 | 58 | Inquiry |
| HEMA PHARMACEUTICALS PVT LTD | +91-9537936912 +91-9537936912 | Gujarat, India | 48 | 58 | Inquiry |
| SURVIVAL TECHNOLOGIES LIMITED | +91 8108285261 | Gujarat, India | 294 | 58 | Inquiry |
| Aptus Therapeutics | +91-9966349855 +91-9966349855 | Telangana, India | 19 | 58 | Inquiry |
| V B Organics | +91-9912996647 +91-9912996647 | Telangana, India | 13 | 58 | Inquiry |
| ORGANO FINE CHEMICALS | +91-91-22-42128300 +91-2242128300 | Mumbai, India | 258 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| BTC pharm India | 58 |
| CLEARSYNTH LABS LTD. | 58 |
| SAGAR LIFE SCIENCES PVT LTD | 58 |
| ANJI BIOSCIENCES | 58 |
| LIFECHEM PHARMA | 58 |
| HEMA PHARMACEUTICALS PVT LTD | 58 |
| SURVIVAL TECHNOLOGIES LIMITED | 58 |
| Aptus Therapeutics | 58 |
| V B Organics | 58 |
| ORGANO FINE CHEMICALS | 58 |
Related articles
- 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride: Synthesis method and uses
- 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride is a white crystalline powder that easily absorbs moisture.
- Apr 16,2024
- 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride: Application, Storage, Safety
- 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, also known as EDC·HCl, is a popular carbodiimide coupling reagent....
- Apr 26,2023
- What is 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride?
- 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide HCl is Commonly known as EDAC, EDC or EDCI, this carbodiimide HCl salt is used a....
- Jul 9,2020
1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride Spectrum
25952-53-8(1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride)Related Search:
1of4
chevron_right




