4-Nitrophthalimide
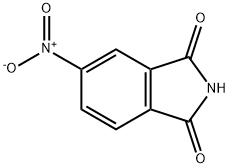
- CAS No.
- 89-40-7
- Chemical Name:
- 4-Nitrophthalimide
- Synonyms
- 5-NITROISOINDOLINE-1,3-DIONE;5-NITROPHTHALIMIDE;AKOS B028733;4-Nitrophtalimide;4-nitro-phthalimid;4-NITROPHTHALIMIDE;4-Nirtophthalimide;P-NITRO-PHTHALIMIDE;PHTHALIMIDE, 4-NITRO-;4-Nitrophthalimide>
- CBNumber:
- CB3681521
- Molecular Formula:
- C8H4N2O4
- Molecular Weight:
- 192.13
- MOL File:
- 89-40-7.mol
- Modify Date:
- 2023/12/25 13:23:17
| Melting point | 206 °C |
|---|---|
| Boiling point | 328.09°C (rough estimate) |
| Density | 1.5513 (rough estimate) |
| refractive index | 1.4900 (estimate) |
| storage temp. | Sealed in dry,Room Temperature |
| form | powder to crystal |
| pka | 7.79±0.20(Predicted) |
| color | Yellow powder |
| Water Solubility | <0.01 g/100 mL at 18 ºC |
| BRN | 180224 |
| Stability | Stable. Combustible. Incompatible with moisture, water, strong oxidising agents, strong bases. |
| InChIKey | ANYWGXDASKQYAD-UHFFFAOYSA-N |
| CAS DataBase Reference | 89-40-7(CAS DataBase Reference) |
| NIST Chemistry Reference | 4-Nitro-phthalimide(89-40-7) |
| EPA Substance Registry System | 4-Nitrophthalimide (89-40-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P261-P264-P271-P280-P302+P352-P305+P351+P338 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 36/37/38 | |||||||||
| Safety Statements | 37/39-26-36-24/25 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | TI5625000 | |||||||||
| Hazard Note | Irritant | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 29251900 | |||||||||
| Toxicity | mmo-sat 333 mg/plate EMMUEG 11(Suppl 12),1,88 | |||||||||
| NFPA 704 |
|
4-Nitrophthalimide price More Price(7)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 332097 | 4-Nitrophthalimide 98% | 89-40-7 | 5G | ₹3626.38 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 332097 | 4-Nitrophthalimide 98% | 89-40-7 | 25G | ₹21736.6 | 2022-06-14 | Buy |
| ALFA India | ALF-A12040-22 | 4-Nitrophthalimide, 98% | 89-40-7 | 100g | ₹23688 | 2022-05-26 | Buy |
| TCI Chemicals (India) | N0247 | 4-Nitrophthalimide | 89-40-7 | 25G | ₹2800 | 2022-05-26 | Buy |
| ALFA India | ALF-A12040-14 | 4-Nitrophthalimide, 98% | 89-40-7 | 25g | ₹8403 | 2022-05-26 | Buy |
4-Nitrophthalimide Chemical Properties,Uses,Production
Chemical Properties
slight yellow powder
Uses
4-Nitrophthalimide is used as intermediate of organic synthesis and fluorescent dyes, it can be used to produce azo dyes, luminol, etc. It can also be used to prepare 4-nitrophthalonitrile, 5-cyanophthalide and other drug intermediates.
Preparation
4-Nitrophthalimide is synthesized from phthalimide by nitration. Cool the fuming nitric acid to below 5°C, slowly add concentrated sulfuric acid dropwise, and control the temperature to 10-13°C. Plus phthalimide. Leave at room temperature overnight. Then, it was poured into ice water with stirring, and the precipitate was precipitated below 20°C. Filter, wash with water, dry, and then recrystallize from ethanol to obtain 4-nitrophthalimide with a yield of 60%.
General Description
Yellow powder.
Air & Water Reactions
4-Nitrophthalimide can be hydrolyzed. . Insoluble in water.
Reactivity Profile
A nitrated amine. Amines are combustible. The combustion of amines yields noxious NOx. REACTIVITY - Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides. Aromatic nitro compounds range from slight to strong oxidizing agents. If mixed with reducing agents, including hydrides, sulfides and nitrides, they may begin a vigorous reaction that culminates in a detonation. The aromatic nitro compounds may explode in the presence of a base such as sodium hydroxide or potassium hydroxide even in the presence of water or organic solvents. The explosive tendencies of aromatic nitro compounds are increased by the presence of multiple nitro groups.
Fire Hazard
Flash point data for 4-Nitrophthalimide are not available, however 4-Nitrophthalimide is probably combustible.
Safety Profile
Mutation data reported. Whenheated to decomposition it emits toxic vapors of NOx.
4-Nitrophthalimide Preparation Products And Raw materials
Raw materials
Preparation Products
1of3
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Danopharm Chemicals Pvt.Ltd. | +91 9821080785 | Mumbai, India | 37 | 58 | Inquiry |
| Hyderabad | +91-9676447615 +91-9676447615 | Telangana, India | 192 | 58 | Inquiry |
| GRINDLAYS PHARMACEUTICALS PRIVATE LIMITED | +91-91-22-25278801 +91-2225278801 | Mumbai, India | 83 | 58 | Inquiry |
| KEVYS GROUP | +91-9000001018 +91-9000001018 | Telangana, India | 23 | 58 | Inquiry |
| JSK Chemicals | +919879767970 | Gujarat, India | 3756 | 58 | Inquiry |
| DL INTRACHEM | +91-9375014678 +91-9099014678 | Gujarat, India | 55 | 58 | Inquiry |
| Paona Chempro Pvt Ltd | +91-2227612326 +91-9904873194 | Maharashtra, India | 7 | 58 | Inquiry |
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| TCI Chemicals (India) Pvt. Ltd. | 1800 425 7889 | New Delhi, India | 6778 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| Danopharm Chemicals Pvt.Ltd. | 58 |
| Hyderabad | 58 |
| GRINDLAYS PHARMACEUTICALS PRIVATE LIMITED | 58 |
| KEVYS GROUP | 58 |
| JSK Chemicals | 58 |
| DL INTRACHEM | 58 |
| Paona Chempro Pvt Ltd | 58 |
| A.J Chemicals | 58 |
| CLEARSYNTH LABS LTD. | 58 |
| TCI Chemicals (India) Pvt. Ltd. | 58 |
89-40-7(4-Nitrophthalimide)Related Search:
1of4
chevron_right




