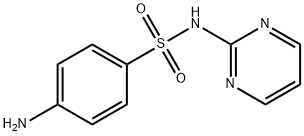
Sulfadiazine
- CAS No.
- 68-35-9
- Chemical Name:
- Sulfadiazine
- Synonyms
- SULPHADIAZINE;Sulfadiazine,(S);SULFADIAZIN;Sulfose;Terfonyl;Diazolone;Neotrizine;Sulfadiazene;SULPHADIAZINE BP;-AMino-N-2-pyriMidinylbenzenesulfonaMide
- CBNumber:
- CB4166214
- Molecular Formula:
- C10H10N4O2S
- Molecular Weight:
- 250.28
- MOL File:
- 68-35-9.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/8/21 22:41:43
| Melting point | 253 °C (dec.) (lit.) |
|---|---|
| Boiling point | 512.6±52.0 °C(Predicted) |
| Density | 1.3780 (rough estimate) |
| refractive index | 1.6440 (estimate) |
| storage temp. | 2-8°C |
| solubility | Soluble in dimethyl sulfoxide. |
| pka | pKa 2.21(H2O t = 25 I = 0.5 (NaCl)) (Uncertain) |
| form | powder |
| color | white |
| Water Solubility | 67.13mg/L(25 ºC) |
| Merck | 14,8903 |
| BRN | 6733588 |
| BCS Class | 4/3 |
| CAS DataBase Reference | 68-35-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Sulfadiazine(68-35-9) |
| EPA Substance Registry System | Benzenesulfonamide, 4-amino-N-2-pyrimidinyl- (68-35-9) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS07,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302-H361d-H362-H411 | |||||||||
| Precautionary statements | P202-P260-P263-P273-P301+P312-P308+P313 | |||||||||
| Hazard Codes | Xn,Xi | |||||||||
| Risk Statements | 22-36/37/38-42/43-43-42 | |||||||||
| Safety Statements | 26-36 | |||||||||
| RIDADR | 3249 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | WP1925000 | |||||||||
| F | 10 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1(b) | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29335990 | |||||||||
| Toxicity | LD50 oral in mouse: 1500mg/kg | |||||||||
| NFPA 704 |
|
Sulfadiazine price More Price(14)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | S8626 | Sulfadiazine 99.0-101.0% | 68-35-9 | 25G | ₹2392.33 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | S8626 | Sulfadiazine 99.0-101.0% | 68-35-9 | 50G | ₹3074.3 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | S8626 | Sulfadiazine 99.0-101.0% | 68-35-9 | 100G | ₹3810.4 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | S8626 | Sulfadiazine 99.0-101.0% | 68-35-9 | 500G | ₹14646.23 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | PHR1343 | Sulfadiazine Pharmaceutical Secondary Standard; Certified Reference Material | 68-35-9 | 500MG | ₹8919.8 | 2022-06-14 | Buy |
Sulfadiazine Chemical Properties,Uses,Production
Chemical Properties
White to slightly yellow crystalline pow
Uses
It is used in the form of silver salts (sulfadiazine silver) as an external antibacterial agent, primarily for treating burns. It is believed that the presence of the silver ion in the molecule facilitates increased antimicrobial and wound-healing action.
Definition
ChEBI: A sulfonamide consisting of pyrimidine with a 4-aminobenzenesulfonamido group at the 2-position.
Antimicrobial activity
Sulfadiazine is somewhat more active than other sulphonamides.
General Description
Sulfadiazine’s plasma half-life is 17 hours. It is a white,odorless crystalline powder soluble in water to the extentof 1:8,100 at 37°C and 1:13,000 at 25°C, in human serumto the extent of 1:620 at 37°C, and sparingly soluble in alcoholand acetone. It is readily soluble in dilute mineralacids and bases. Its pKa is 6.3.
Pharmaceutical Applications
Sulfadiazine is almost insoluble in water and unstable on exposure to light. It is administered orally or, as the sodium salt, by intravenous injection. It is a component of several multi-ingredient preparations. Its low solubility in urine led to its general replacement by other compounds. The intravenous solution is highly alkaline and should not be given by any other route.
Pharmacokinetics
Oral absorption: Very good
Cmax 3 g oral: c. 50 mg/L after 3–4 h
Plasma half-life :7–12 h
Volume of distribution: 0.36 L/kg
Plasma protein binding: c. 40%
Absorption and distribution
Adequate blood concentrations are easily achieved and
maintained after oral administration. It is well distributed
and penetrates in therapeutic concentrations into
the CSF, but because of resistance it is no longer the
drug of choice in meningitis. It crosses the placenta and
enters breast milk to achieve concentrations around 20%
of plasma levels.
Metabolism and excretion
Sulfadiazine is subject to acetylation in the liver. The acetyl
derivative lacks antibacterial activity and is excreted more
slowly (half-life 8–18 h). Parent compound and metabolite
are both excreted mainly by glomerular filtration.
Clinical Use
Urinary tract infection
Nocardiasis
Chancroid
Toxoplasmosis (in combination with pyrimethamine)
Meningococcal infections
Prophylaxis of rheumatic fever
Side effects
In addition to side effects common to the group, sulfadiazine inhibits the metabolism of phenytoin. The risk of crystalluria can be reduced by high fluid intake and alkalization of the urine.
Safety Profile
Poison by intravenous route. Moderately toxic by ingestion and intraperitoneal routes. Human systemic effects by ingestion: hematuria, anuria, general anesthesia, gastrointestinal effects. Experimental teratogenic and reproductive effects. When heated to decomposition it emits very toxic fumes of NOx and SOx.
Sulfadiazine Preparation Products And Raw materials
Raw materials
1of3
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Medi Pharma Drug House | +919930911911 | Mumbai, India | 143 | 58 | Inquiry |
| Virchow Groups | +919394860022 | Telangana, India | 24 | 58 | Inquiry |
| TCI Chemicals (India) Pvt. Ltd. | 1800 425 7889 | New Delhi, India | 6778 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| Alfa Aesar | 1 800 209 7001 | Maharashtra, India | 6913 | 58 | Inquiry |
| Sisco Research Laboratories Pvt. Ltd. | +91-22-4268 5800 | Mumbai, India | 4317 | 58 | Inquiry |
| Central Drug House(P) Ltd. | 91-11-49404040 | New Delhi, India | 6160 | 58 | Inquiry |
| SynZeal Research Pvt Ltd | +1 226-802-2078 | Gujarat, India | 6522 | 58 | Inquiry |
| Triveni chemicals | 08048762458 | New Delhi, India | 6093 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| Medi Pharma Drug House | 58 |
| Virchow Groups | 58 |
| TCI Chemicals (India) Pvt. Ltd. | 58 |
| CLEARSYNTH LABS LTD. | 58 |
| A.J Chemicals | 58 |
| Alfa Aesar | 58 |
| Sisco Research Laboratories Pvt. Ltd. | 58 |
| Central Drug House(P) Ltd. | 58 |
| SynZeal Research Pvt Ltd | 58 |
| Triveni chemicals | 58 |





