Cefixime
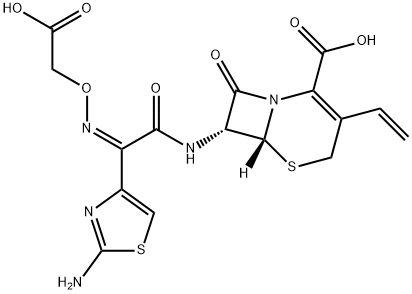
- CAS No.
- 79350-37-1
- Chemical Name:
- Cefixime
- Synonyms
- CEFIXIME TRIHYDRATE;Cefixim;Cefspan;Suprax;Cefixime USP;CEFIXIME MICRONIZED;CFIX;fk027;Oroken;Saprax
- CBNumber:
- CB4210362
- Molecular Formula:
- C16H15N5O7S2
- Molecular Weight:
- 453.45
- MOL File:
- 79350-37-1.mol
- MSDS File:
- SDS
- Modify Date:
- 2025/4/22 20:29:20
| Melting point | 218-225°C |
|---|---|
| Density | 1.85±0.1 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Inert atmosphere,2-8°C |
| solubility | Slightly soluble in water, soluble in methanol, sparingly soluble in anhydrous ethanol, practically insoluble in ethyl acetate. |
| pka | pKa 2.10 (Uncertain) |
| form | Solid |
| color | Off-White to Pale Yellow |
| Water Solubility | 55.11 mg/L |
| BCS Class | 4 |
| Stability | Hygroscopic |
| CAS DataBase Reference | 79350-37-1(CAS DataBase Reference) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H335 |
| Precautionary statements | P261-P305+P351+P338 |
| Hazard Codes | Xn |
| Risk Statements | 42/43 |
| Safety Statements | 22-36/37-45 |
| WGK Germany | 3 |
| F | 10-23 |
| HS Code | 38210000 |
| Toxicity | LD50 oral in rat: > 10gm/kg |
Cefixime Chemical Properties,Uses,Production
Description
Cefixime is the first orally active third-generation cephalosponn with a true broad spectrum of activity. It is reportedly more effective against Gram-negative bacteria than conventional oral cephems such as cephalexin and cefaclor.
Chemical Properties
Cefixime is an oral third generation cephalosporin antibiotic. It was sold under the trade name Suprax in the United States until 2003. The oral suspension form of “Suprax” was re-launched. Cefixime is prescribed for bacterial infections of the chest, ears, urinary tract, and throat (tonsilitis and pharyngitis), and for uncomplicated gonorrhea, upper and lower respiratory tract infections, acute otitis media, and gonococcal urethritis.
Definition
ChEBI: A third-generation cephalosporin antibiotic bearing vinyl and (2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-[(carboxymethoxy)imino]acetamido groups at positions 3 and 7, respectively, of the cephem skeleton. It is used in the treatment of gonorrhoea tonsilitis, pharyngitis, bronchitis, and urinary tract infections.
Antimicrobial activity
It is active against N. gonorrhoeae, M. catarrhalis, H. influenzae and a wide range of enterobacteria, including most strains of Citrobacter, Enterobacter and Serratia spp. Its antistaphylococcal activity is poor. It is not active against Acinetobacter spp., Ps. aeruginosa or B. fragilis. It is resistant to hydrolysis by common β-lactamases.
Health Hazard
Exposures to cefi xime may cause side effects that include, but are not limited to, stomach and abdominal pain, diarrhea, vomiting, mild skin rash, headache, fever, urticaria, pruritis, eosinophilia, leucopenia, anaphylaxis, superinfection, hemolytic anemia, dyspepsia, and fl atulence. Also, cefi xime may cause transient elevation of SGOT, SGPT, alkaline phosphatase, BUN, and creatinine. Reports have indicated that cefi xime is mainly excreted unchanged in the bile and urine
Pharmacokinetics
Oral absorption: c. 50%
Cmax 400 mg oral: 4–5.5 mg/L after 4 h
Plasma half-life: 3-ndash;4 h
Volume of distribution: 0.1 L/kg
Plasma protein binding: 60-ndash;70%
Absorption and distribution
Oral absorption is slow and incomplete, but is unaffected by aluminum magnesium hydroxide. Penetration into cantharides blister fluid was very slow but exceeded the plasma level. CSF concentrations are poor even in the presence of meningeal inflammation, reaching an average of around 0.22 mg/L in children with meningitis.
Metabolism and excretion
It is not metabolized and is excreted unchanged in urine (mainly by glomerular filtration) and in bile, in which concentrations exceeding 100 mg/L have been found. Less than 20% of an oral dose is recovered from the urine over 24 h, falling to less than 5% in patients with severe renal impairment, with a corresponding increase in plasma concentration. It is not removed by peritoneal or hemodialysis.
Clinical Use
Cefixime has been used successfully in uncomplicated cystitis, upper and lower respiratory tract infections and various other infections. Its failure to provide adequate cover for staphylococci should be noted.
Side effects
It is well tolerated, but diarrhea is fairly common and pseudomembranous colitis has been reported. Other side effects common to cephalosporins are occasionally seen.
Precautions
Users of cefi xime should be careful in health conditions such as neonates, pregnancy, lactation, and renal failure. Users should follow suitable and appropriate health care measures to control superinfection (if it happens during therapy). It is contraindicated in patients with a known allergy to penicillin or any other ingredients of Taximax. Taxim-O is contraindicated in patients with a known allergy to the cephalosporin group of antibiotics.
Cefixime Preparation Products And Raw materials
Raw materials
1of3
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Hetero Drugs Limited | +91-4023704923 +91-4023704923 | Telangana, India | 296 | 58 | Inquiry |
| Covalent Laboratories Private Limited (Virchow Group) | +91-4049483333 +91-9866077161 | Telangana, India | 9 | 58 | Inquiry |
| Concept Pharmaceuticals Ltd | +91-2242418888 +91-2242418888 | Maharashtra, India | 19 | 58 | Inquiry |
| Unimark Remedies Ltd | +91-2267304120 +91-2267304120 | Maharashtra, India | 48 | 58 | Inquiry |
| Ralington Pharma | +91-7948911722 +91-9687771722 | Gujarat, India | 1350 | 58 | Inquiry |
| APITORIA PHARMA PRIVATE LIMITED | +91 40 6672 5000 | Chongqing, India | 159 | 58 | Inquiry |
| APITORIA PHARMA PRIVATE LTD | +91 40 6672 5000 | Chongqing, India | 110 | 58 | Inquiry |
| Shree Nath Life Sciences Pvt., Ltd. | 91-11-23864528 | Delhi, India | 15 | 58 | Inquiry |
| Aligns International | 91-22-22634745 | Maharashtra, India | 137 | 58 | Inquiry |
| Shivam Pharma Chemicals | 91-22-26403900 | Maharashtra, India | 656 | 58 | Inquiry |
Related articles
- The use of Cefixime for childhood infections
- Cefixime is a broad-spectrum, third-generation cephalosporin antibiotic derived semisynthetically from the marine fungus Cepha....
- Sep 5,2024
- Cefixime: Antimicrobial Activity, Susceptibility, Administration and Dosage, Clinical Uses etc.
- Cefixime is an orally administered cephalosporin with a broader antimicrobial spectrum than those of earlier oral cephalospori....
- Mar 24,2022





