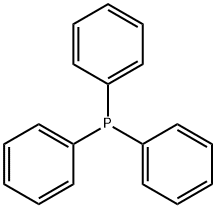
Triphenylphosphine
- CAS No.
- 603-35-0
- Chemical Name:
- Triphenylphosphine
- Synonyms
- PPh3;Triphenylphosphane;Triphenyl phosphine;Triphenyl;Triphenyphosphine;triphenyl-phosphane;TRIPHENYLPHOSPHORUS;LPO Assay Triphenylphosphine;PP-360;'LGC' (4006)
- CBNumber:
- CB8771461
- Molecular Formula:
- C18H15P
- Molecular Weight:
- 262.29
- MOL File:
- 603-35-0.mol
- MSDS File:
- SDS
- Modify Date:
- 2025/1/27 9:38:02
| Melting point | 79-81 °C(lit.) |
|---|---|
| Boiling point | 377 °C(lit.) |
| bulk density | 500-600kg/m3 |
| Density | 1.132 |
| vapor density | 9 (vs air) |
| vapor pressure | 5 mm Hg ( 20 °C) |
| refractive index | 1.6358 |
| Flash point | 181 °C |
| storage temp. | Store below +30°C. |
| solubility | water: soluble0.00017 g/L at 22°C |
| form | Crystals, Crystalline Powder or Flakes |
| color | White |
| Specific Gravity | 1.132 |
| Odor | odorless |
| Water Solubility | Insoluble |
| Hydrolytic Sensitivity | 8: reacts rapidly with moisture, water, protic solvents |
| Merck | 14,9743 |
| BRN | 610776 |
| Stability | Stable. Incompatible with oxidizing agents, acids. |
| InChIKey | RIOQSEWOXXDEQQ-UHFFFAOYSA-N |
| CAS DataBase Reference | 603-35-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Phosphine, triphenyl-(603-35-0) |
| EPA Substance Registry System | Triphenylphosphine (603-35-0) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS05,GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H302-H317-H318-H372 | |||||||||
| Precautionary statements | P260-P280-P301+P312-P302+P352-P305+P351+P338-P314 | |||||||||
| Hazard Codes | Xn,N | |||||||||
| Risk Statements | 22-43-53-50/53-48/20/22 | |||||||||
| Safety Statements | 36/37-60-61-36/37/39-26 | |||||||||
| RIDADR | 3077 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | SZ3500000 | |||||||||
| F | 9 | |||||||||
| Autoignition Temperature | 425 °C | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 29310095 | |||||||||
| Hazardous Substances Data | 603-35-0(Hazardous Substances Data) | |||||||||
| Toxicity | LD50 orally in Rabbit: 700 mg/kg LD50 dermal Rabbit > 4000 mg/kg | |||||||||
| NFPA 704 |
|
Triphenylphosphine price More Price(29)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | T84409 | Triphenylphosphine ReagentPlus?, 99% | 603-35-0 | 1G | ₹2200 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | T84409 | Triphenylphosphine ReagentPlus?, 99% | 603-35-0 | 25G | ₹2490 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | T84409 | Triphenylphosphine ReagentPlus?, 99% | 603-35-0 | 100G | ₹3130 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | T84409 | Triphenylphosphine ReagentPlus?, 99% | 603-35-0 | 500G | ₹13834.35 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | T84409 | Triphenylphosphine ReagentPlus?, 99% | 603-35-0 | 1KG | ₹14029.2 | 2022-06-14 | Buy |
Triphenylphosphine Chemical Properties,Uses,Production
Description
Triphenylphosphine: a member of tertiary phosphines
Triphenylphosphine (TPP) is a member of tertiary phosphines, which is phosphane, in which the three hydrogens are replaced by phenyl groups. It has a role as a reducing agent and an NMR chemical shift reference compound. It is a crucial ligand utilized in the Wittig reaction for alkene synthesis. This reaction involves the formation of alkyliden-etriphenylphosphoranes from the action of butyllithium or another base on the quarternary halide. Triphenylphosphine is used to synthesise organic compounds due to its nucleophilicity and reducing character.
Chemical Properties
Triphenylphosphine is a white to light tan flaked solid. Insoluble inwater; slightly soluble in alcohol; soluble in benzene, acetone, carbon tetrachloride. Combustible.
Uses
Triphenylphosphine is a versatile and efficient compound with a wide range of applications. It serves as a crucial ligand in homogeneous catalysts for petrochemical and fine chemical production, and as a co-catalyst in the production of isobutanol and n-butanol. It is also the basic raw material for rhodium phosphine complex catalysts, such as Wilkinson's catalyst (RhCl(PPh3)3) for alkene hydrogenation and tetrakis(triphenylphosphine)palladium(0) for C-C coupling reactions in organic synthesis. In the dye industry, it is utilized as a sensitizer, heat stabilizer, light stabilizer, antioxidant, flame retardant, antistatic agent, rubber antiozonant, and analytical reagent. The rhodium and triphenylphosphine catalyst system is employed in the hydroformylation of vegetable oils and their methyl esters, and polymer-supported triphenylphosphine catalyzes the γ-addition of pronucleophiles to alkynoates. It also participates in the Heck reaction of 4-bromoanisole and ethyl acrylate in ionic liquids. Triphenylphosphine can be sulfonated to form trisulfonic acid and is used in Wittig synthesis as a standard ligand in homogeneous catalysis. It is involved in the synthesis of trimethyl phosphite, leading to the production of organophosphorus pesticides like dichlorvos, monocrotophos, and phosphamidon. Furthermore, it is used as a stabilizer in rubber and resin synthesis, an antioxidant in polyvinyl chloride, and a raw material in the synthesis of alkyd resins and polyester resins. Triphenylphosphine is also used in the synthesis of Chlorambucil, a cytotoxic agent for breast and pancreatic cancers, and in the preparation of α-Tocopherol analogues for monitoring antioxidant status.
Production Methods
Triphenylphosphine is one of the most widely used phosphorus-containing reagents in organic synthesis for many types of transformations such as the Mitsunobu, the Wittig, and the Staudinger reaction. Triphenylphosphine can be prepared in the laboratory by treatment of phosphorus trichloride with phenylmagnesium bromide or phenyllithium. The industrial synthesis involves the reaction between phosphorus trichloride, chlorobenzene, and sodium.
PCl3 + 3 PhCl + 6 Na → PPh3 + 6 NaCl
Reactivity Profile
Triphenylphosphine reacts vigorously with oxidizing materials. .
Health Hazard
ACUTE/CHRONIC HAZARDS: Toxic; when heated to decomposition, emits highly toxic fumes of phosphine and POx.
Safety Profile
Moderately toxic by ingestion. Mildly toxic by inhalation. A skin and eye irritant. Combustible when exposed to heat or flame. Slight explosion hazard in the form of vapor when exposed to flame. Can react vigorously with oxidizing materials. To fight fire, use dry chemical, fog, CO2. When heated to decomposition it emits highly toxic fumes of phosphne and POx. See also PHOSPHINE and PHENOL.
Purification Methods
It crystallises from hexane, MeOH, diethyl ether, CH2Cl2/hexane or 95% EtOH. Dry it at 65o/<1mm over CaSO4 or P2O5. Chromatograph it through alumina using (4:1) *benzene/CHCl3 as eluent. [Blau & Espenson et al. J Am Chem Soc 108 1962 1986, Buchanan et al. J Am Chem Soc 108 1537 1986, Randolph & Wrighton J Am Chem Soc 108 3366 1986, Asali et al. J Am Chem Soc 109 5386 1987.] It has also been crystallised twice from pet ether and 5 times from Et2O/EtOH to give m 80.5o. Alternatively, dissolve it in conc HCl, and upon dilution with H2O it separates because it is weakly basic, it is then crystallised from EtOH/Et2O. It recrystallises unchanged from AcOH. [Forward et al. J Chem Soc Suppl. p121 1949, Muller et al. J Am Chem Soc 78 3557 1956.] 3Ph3P.4HCl crystallises out when HCl gas is bubbled through an Et2O solution, it has m 70-73o, but recrystallises very slowly and is deliquescent. The hydriodide, made by adding Ph3P to hydriodic acid, is not hygroscopic and decomposes at ~100o. The chlorate (1:1) salt has m 165-167o, but decomposes slowly at 100o. All salts hydrolyse in H2O to give Ph3P [IR, UV: Sheldon & Tyree J Am Chem Soc 80 2117 1958, pK: Henderson & Streuli J Am Chem Soc 82 5791 1960, Kosolapoff, Organophosphorus Compounds, Wiley 1950]. [Beilstein 16 IV 951.] § Available commercially on a polystyrene or polyethyleneglycol support.
Triphenylphosphine Preparation Products And Raw materials
Raw materials
Preparation Products
1of8
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| GLR Innovations | +91 9891111994 | New Delhi, India | 4535 | 58 | Inquiry |
| V B Organics | +91-9912996647 +91-9912996647 | Telangana, India | 13 | 58 | Inquiry |
| VGS SYNTHESIS PRIVATE LIMITED | +91-9866975588 +91-9866975588 | Hyderabad, India | 1817 | 58 | Inquiry |
| SYNGENESIS CHEMSCIENCE PRIVATE LIMITED | +91-9067779401 +91-9067779401 | Maharashtra, India | 58 | 58 | Inquiry |
| GVSK PHARMA PVT LTD | +91-9492952000 +91-9492951000 | Hyderabad, India | 11 | 58 | Inquiry |
| Otto Chemie Pvt Ltd | +91-2222070099 +91-2266382599 | Maharashtra, India | 429 | 58 | Inquiry |
| BASF India Limited | +91-2262785600 +91-2262785600 | Maharashtra, India | 209 | 58 | Inquiry |
| Virchow Groups | +919394860022 | Telangana, India | 24 | 58 | Inquiry |
| Roopa Industries Limited | +91-8096330007 +91-9246216128 | Telangana, India | 11 | 58 | Inquiry |
| Aether Industries Limited | +91-261-6603130 +91-2616603130 | Gujarat, India | 145 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| GLR Innovations | 58 |
| V B Organics | 58 |
| VGS SYNTHESIS PRIVATE LIMITED | 58 |
| SYNGENESIS CHEMSCIENCE PRIVATE LIMITED | 58 |
| GVSK PHARMA PVT LTD | 58 |
| Otto Chemie Pvt Ltd | 58 |
| BASF India Limited | 58 |
| Virchow Groups | 58 |
| Roopa Industries Limited | 58 |
| Aether Industries Limited | 58 |
Related articles
- Convenient Synthesis of Triphenylphosphine Sulfide from Sulfur and Triphenylphosphine
- Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the chemical formula P(C6H5)3, ....
- Apr 16,2024
- Triphenylphosphine: Applications, Synthesis, Storage,Catalytic mechanism, toxicity
- Triphenylphosphine is a common organophosphorus compound with the formula (C6H5)3P. It is a colorless crystalline solid, which....
- Apr 26,2023
- Applications of Triphenylphosphine
- Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 - often ab....
- Nov 6,2019
603-35-0(Triphenylphosphine)Related Search:
1of4
chevron_right




