Suramin sodium
- CAS No.
- 129-46-4
- Chemical Name:
- Suramin sodium
- Synonyms
- SURAMIN;SURAMIN HEXASODIUM SALT;SURAMIN SODIUM SALT;nf060;reasodiumsalt;suraminesodium;naphuridesodium;Suramin, Sodium Salt - CAS 129-46-4 - Calbiochem;309f;F-309
- CBNumber:
- CB4334789
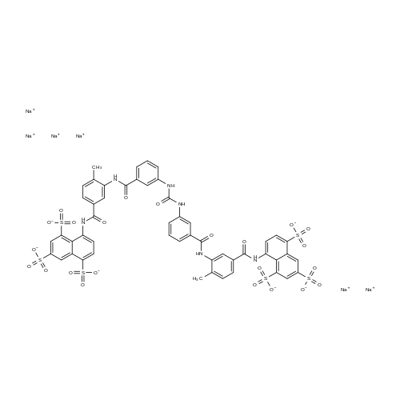
- Molecular Formula:
- C51H34N6Na6O23S6
- Molecular Weight:
- 1429.17
- MOL File:
- 129-46-4.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/5/30 19:23:35
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H317 |
| Precautionary statements | P261-P272-P280-P302+P352-P333+P313-P321-P363-P501 |
| Safety Statements | 22-24/25 |
| WGK Germany | 3 |
| RTECS | QM7000000 |
| F | 3-10 |
| HS Code | 29242998 |
| Toxicity | LD50 in mice (mg/kg): ~620 i.v. (Hawking) |
Suramin sodium price More Price(3)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | S2671 | Suramin sodium salt ≥98% (TLC) | 129-46-4 | 25MG | ₹8595.05 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | S2671 | Suramin sodium salt ≥98% (TLC) | 129-46-4 | 100MG | ₹14559.63 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 574625 | Suramin, Sodium Salt - CAS 129-46-4 - Calbiochem A reversible and competitive inhibitor of protein tyrosine phosphatases. | 129-46-4 | 50MG | ₹4589.99 | 2022-06-14 | Buy |
Suramin sodium Chemical Properties,Uses,Production
Description
Introduced into therapy for the treatment of early trypanosomiasis in the 1920s, suramin, a bis-hexasulfonatednaphthylurea, is still considered to be the drug of choice for treatment of non-CNS-associated African trypanosomiasis.
Chemical Properties
White crystalline powder
Uses
Suramin sodium is a compound with a dyelike structure. Suramin is most effective against T. b. rhodesiense, but has also been used against T. b. gambiense infection. The compound causes side effects such as nausea, photophobia, and peripheral neuropathy which disappear shortly after conclusion of administration. Because the drug is unable to pass the bloodbrain barrier, prompt treatment of patients is essential. Suramin in combination with tryparsamide is an alternative that has been investigated.
Indications
Suramin (Germanin) is a derivative of a nonmetallic dye whose antiparasitic mechanism of action is not clear. It appears to act on parasite specificα-glycerophosphate oxidase, thymidylate synthetase, dihydrofolate reductase, and protein kinase but not on host enzymes.
Antimicrobial activity
Suramin has no significant trypanocidal activity in vitro, but is effective in animals infected with T. brucei. Trypanosomes take up suramin bound to plasma protein by a combination of fluid phase and receptor-mediated endocytosis. It acts synergistically with nitroimidazoles and eflornithine in the elimination of trypanosomes from CSF of infected mice.
Acquired resistance
Relapse rates of 30–50% have been recorded in Kenya and Tanzania but there is no evidence of resistant parasites. Stable resistance has been described in the related camel parasite Trypanosoma evansi.
Pharmaceutical Applications
A complex symmetrical molecule originally developed in Germany in the early 1920s for the treatment of African trypanosomiasis. Its useful anthelmintic activity is restricted to O. volvulus and it has been used to achieve a radical cure of onchocerciasis by killing the adult worms. However, it is an extremely toxic drug and its use has become increasingly uncommon since ivermectin became available.
Biological Activity
Non-selective P2 purinergic antagonist. Also blocks calmodulin binding to recognition sites and G protein coupling to G protein-coupled receptors. Anticancer and antiviral agent.
Mechanism of action
Suramin is not absorbed from the intestinal tract and is administered intravenously. Although the initial high plasma levels drop rapidly, suramin binds tightly to and is slowly released from plasma proteins, and so it persists in the host for up to 3 months. Suramin neither penetrates red blood cells nor enters the CNS. It is taken up by the reticuloendothelial cells and accumulates in the Kupffer cells of the liver and in the epithelial cells of the proximal convoluted tubules of the kidney. It is excreted by glomerular filtration, largely as the intact molecule.
Pharmacokinetics
Oral absorption: Poor
Cmax 1 g intravenous doses (6 doses
at weekly intervals):
100 mg/L
Plasma half-life: 44–54 days
Volume of distribution: 20–80 L
Plasma protein binding: >99%
It is normally administered by slow intravenous infusion. It can
be detected in blood for 3 months; plasma levels >100 mg/L
were observed for several weeks after a 6-week course of treatment.
No metabolism was observed and 80% was removed
by renal clearance. Distribution to mononuclear phagocytes,
especially liver macrophages, the adrenal glands and the kidney
is high. It does not enter erythrocytes and penetrates the
blood–brain barrier poorly.
Clinical Use
Suramin sodium is a high molecular-weight bisurea derivative containing six sulfonic acid groups as their sodium salts. It was developed in Germany shortly after World War I as a byproduct of research efforts directed toward the development of potential antiparasitic agents from dyestuffs. The drug has been used for more than half a century for the treatment of early cases of trypanosomiasis. Not until several decades later, however, was suramin discovered to be a long-term prophylactic agent whose effectiveness after a single intravenous injection is maintained for up to 3 months. The drug is tightly bound to plasma proteins, causing its excretion in the urine to be almost negligible. Tissue penetration of the drug does not occur, apparently because of its high molecular weight and highly ionic character. Thus, an injected dose remains in the plasma for a very long period. Newer, more effective drugs are now available for short-term treatment and prophylaxis of African sleeping sickness. Suramin is also used for prophylaxis of onchocerciasis. It is available from the CDC.
Side effects
Suramin is toxic, especially in malnourished patients. A test dose of 200 mg has been recommended. Immediate febrile reactions (nausea, vomiting, loss of consciousness) can be avoided by slow intravenous administration. Intramuscular or subcutaneous injections are painful and irritating, and can be followed by fever and urticaria. Anaphylactic shock occurs in fewer than 1 in 2000 patients. Delayed reactions include renal damage, exfoliative dermatitis, anemia, leukopenia, agranulocytosis, jaundice and diarrhea.
Suramin sodium Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Neugen Labs | +91-8133254502 +91-8133254502 | Karnataka, India | 195 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| CHEMSWORTH | +91-261-2397244 | New Delhi, India | 6707 | 30 | Inquiry |
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| Neugen Labs | 1--8133254502 | Karnataka, India | 99 | 58 | Inquiry |
| Pharma Affiliates | 172-5066494 | Haryana, India | 6761 | 58 | Inquiry |
| Shandong Natural Micron Pharm Tech Co.,LTD | +86-18653895227 | China | 94 | 58 | Inquiry |
| Hebei Kingfiner Technology Development Co.Ltd | +86-15532196582 +86-15373005021 | China | 2989 | 58 | Inquiry |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | China | 2503 | 58 | Inquiry |
Related articles
- Side effects of Suramin
- Suramin is used in the treatment of African sleeping sickness (African trypanosomiasis) and river blindness (onchocerciasis), ....
- Jul 15,2022
129-46-4(Suramin sodium)Related Search:
1of4
chevron_right




