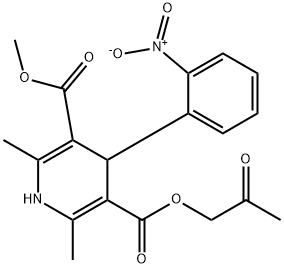
ARANIDIPINE
- CAS No.
- 86780-90-7
- Chemical Name:
- ARANIDIPINE
- Synonyms
- Sapresta;MPC-1304;ARANIDIPINE;Asanidipine;Aranidipine MPC1304;ARANIDIPINE USP/EP/BP;ARANIDIPINE MPC1304;MPC-1304;MPC 1304;Calcium Channel,inhibit,MPC 1304,Inhibitor,MPC-1304,Aranidipine,Ca channels,Ca2+ channels;3-Methyl 5-(2-oxopropyl) 2,6-diMethyl-4-(2-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate;2,6-Dimethyl-4-(2-nitrophenyl)-1,4-dihydro-3,5-pyridinedicarboxylic acid methyl 2-oxopropyl ester
- CBNumber:
- CB4386708
- Molecular Formula:
- C19H20N2O7
- Molecular Weight:
- 388.37
- MOL File:
- 86780-90-7.mol
- Modify Date:
- 2024/7/2 8:55:09
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302+H312+H332 |
| Precautionary statements | P261-P264-P270-P271-P280-P301+P312+P330-P302+P352+P312+P362+P364-P304+P340+P312-P501 |
| Toxicity | LD50 in male, female mice, rats (mg/kg): 143, 193, 1982, 1459 orally; LD50 in male, female mice (mg/kg): 7.3, 9.1 i.p. (Nakano) |
ARANIDIPINE Chemical Properties,Uses,Production
Description
Aranidipine was launched in Japan as an antihypertensive agent. Its pharmacological effects are similar to other dihydropyridine derivatives, e.g., nefidipine, however, it is more potent and longer lasting. This is partially due to the fact that its initial metabolite (ketone reduction) is just as effective as the parent compound. Aranidipine exerts its activity by blocking Ca+2 entry during depolarization via L-type voltagegated Ca channels. This causes decreased levels of intracellular Ca which leads to enhanced relaxation of smooth and cardiac muscle. It is a selective α2-adrenoreceptor antagonist which inhibits vasoconstrictive responses. As a dihydropyridine derivative, it can be synthesized via a modified Hantsch synthesis. While sold as a racemate, the (S)-enantiomer is 150 times more active than the (R)- antipode.
ARANIDIPINE Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | China | 32760 | 60 | Inquiry |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | China | 9030 | 58 | Inquiry |
| Dayang Chem (Hangzhou) Co.,Ltd. | 571-88938639 +8617705817739 | China | 52861 | 58 | Inquiry |
| InvivoChem | +1-708-310-1919 +1-13798911105 | United States | 6393 | 58 | Inquiry |
| TargetMol Chemicals Inc. | +1-781-999-5354 | United States | 19973 | 58 | Inquiry |
| PT CHEM GROUP LIMITED | +86-85511178 +86-85511178 | China | 35451 | 58 | Inquiry |
| Guizhou Dida Technology Co., Ltd | 0851-8475-2296 18096062265 | China | 2779 | 58 | Inquiry |
| Beijing Solarbio Science & Tecnology Co., Ltd. | 010-50973130 4009686088 | China | 29795 | 68 | Inquiry |
| TargetMol Chemicals Inc. | 4008200310 | China | 24017 | 58 | Inquiry |
| Jinan Jiuli Biotechnology Co. , Ltd. | 14768611851 | China | 2041 | 58 | Inquiry |





