Metalaxyl
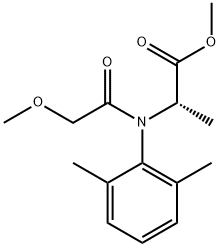
- CAS No.
- 57837-19-1
- Chemical Name:
- Metalaxyl
- Synonyms
- SPAN;METALAXIL;Acylon;Jiashuangling;N-(2,6-Dimethylphenyl)-N-(methoxyacetyl)-dl-alanine methyl ester;Bleu;axyL;Fubol;MILOR;TOKAT
- CBNumber:
- CB4431035
- Molecular Formula:
- C15H21NO4
- Molecular Weight:
- 279.33
- MOL File:
- 57837-19-1.mol
- Modify Date:
- 2024/7/2 8:55:09
| Melting point | 72-73°C |
|---|---|
| Boiling point | 422.1°C (rough estimate) |
| Density | 1.1083 (rough estimate) |
| vapor pressure | 7.5 x 10-4 Pa (25 °C) |
| refractive index | 1.5130 (estimate) |
| Flash point | 100 °C |
| storage temp. | Sealed in dry,2-8°C |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 1.41±0.50(Predicted) |
| color | Off-White to Pale Beige |
| Water Solubility | 0.84 g/100 mL |
| BRN | 2947777 |
| CAS DataBase Reference | 57837-19-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Dl-alanine, n-(2,6-dimethylphenyl)-n-(methoxyacetyl)-, methyl ester(57837-19-1) |
| EPA Substance Registry System | Metalaxyl (57837-19-1) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302-H317-H412 | |||||||||
| Precautionary statements | P261-P264-P273-P280-P301+P312-P302+P352 | |||||||||
| Hazard Codes | Xn,F | |||||||||
| Risk Statements | 22-43-52/53-36-20/21/22-11 | |||||||||
| Safety Statements | 13-24-37-46-61-36-26-16 | |||||||||
| RIDADR | UN 1648 3/PG 2 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | AY6910000 | |||||||||
| HS Code | 29242990 | |||||||||
| Toxicity | LD50 in rats (mg/kg): 669 orally (Urech) | |||||||||
| NFPA 704 |
|
Metalaxyl price More Price(2)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 89979 | Metalaxyl certified reference material, TraceCERT? | 57837-19-1 | 50MG | ₹21942.28 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 32012 | Metalaxyl PESTANAL?, analytical standard | 57837-19-1 | 100MG | ₹5564.05 | 2022-06-14 | Buy |
Metalaxyl Chemical Properties,Uses,Production
Description
As an important fungicide group, acylanilides have a longer history than triazoles. Metalaxyl (44) is one of the most important fungicides in the group. It originates from the class of herbicidal chloroacetanilides, particularly metolachlor (45). Metolachlor, having a chiral carbon center and a chiral axis, is composed of four stereoisomers, the most herbicidally active one of which is the (αR, 1S)-isomer (32). For the stereoselective synthesis of this isomer, novel iridium-ferrocenyldiphosphine catalysts for the enantioselective hydrogenation of N-(2-ethyl-6-methylphenyl)- N-(1 -methoxymethyl)-ethylidene-amine are reported. In the most effective approach, the catalyst is generated in situ from [Ir(cod)Cl]2(cod = cyclooctadiene) and the ferrocenyldiphosphine ligand (R)-(S)-PPF-P(3,5-xyl)2 (46) (33).When the substrate to catalyst ratio is 106, the conversion is complete in 2 to 3 h, producing metolachlor in optical yields over 80% (34).
Chemical Properties
Pale Beige Solid
Uses
Metalaxyl is used for the control of air-borne pathogens by foliar application and of soil-borne pathogens by soil application on a wide range of crops. It is particularly useful against Oomycetes including soil-borne Phytophthora diseases.
Hazard
Moderately toxic by ingestion.
Agricultural Uses
Fungicide: Metalaxyl is used as a systemic fungicide on a variety of food and non-food crops including tobacco, turf and conifers, and ornamentals. Used in combination with fungicides of different mode of action as a foliar spray on tropical and subtropical crops; as a seed treatment to control downy mildew; and as a soil fumigant to control soilborne pathogens.
Trade name
AGROX® PREMIERE; ALLEGIENCE®; APRON®; CG 117®; CGA-48988®; CHLORAXYL®; COTGUARD®; EPERON®; DELTA-COAT; FOLIO® GOLD; GAUCHO®; KODIAK®; METALAXIL®; METAXANIN®; PACE®; PREVAIL®; RAXIL® (tebu- conazole + metalaxyl); RIDOMIL® GOLD/BRAVO®; RIDOMIL®; RIDOMIL 2E®; SUBDUE®
Pharmacology
In mycelium of Phytophthora
megasperma,metalaxyl affected primarily rRNA synthesis
(polymerase I), whereas mRNA was much less sensitive;
therefore, inhibition of rRNA synthesis is considered as
the primary site of action of PAFs (23).
The PAFs exhibit strong preventive and curative
activity. They affect especially hyphal growth (inside and
outside the plant tissue) as well as haustorium and spore
formation (15). Although not fully utilized for resistance
management reasons, PAFs also exhibit strong eradicative
and antisporulant activity in the disease cycle of target
pathogens. On the other hand, PAFs do not inhibit the
early stages in the disease cycle like zoospore release,
spore germination, and penetration of the host tissue (15).
Because spores contain many ribosomes to support early
growth stages, RNA synthesis is fully operating only
after spore germination; later development stages are
therefore most sensitive to PAFs (23). As a consequence
of RNA inhibition, the precursors of RNA synthesis (i.e.,
nucleoside triphosphates) are accumulated; they activate
β-1,3-glucansynthetases, which are involved in cell wall
formation (23). Metalaxyl-treated hyphae often produce
thicker cell walls than do untreated ones.
Safety Profile
Moderately toxic by ingestion. When heated to decomposition it emits toxic fumes of NOx.
Potential Exposure
Metalaxyl is phenylamide systemic fungicide used on a variety of food and nonfood crops including tobacco, turf and conifers, and ornamentals. Used in combination with fungicides of different mode of action as a foliar spray on tropical and subtropical crops; as a seed treatment to control downy mildew; and as a soil fumigant to control soil-borne pathogens. Banned for use in EU.
Environmental Fate
Soil. Little information is available on the degradation of metalaxyl in soil; however,
Sharom and Edgington (1986) reported metalaxyl acid as a possible metabolite. Repeated
applications of metalaxyl decreases its persistence. Following an initial application, the
average half-life was 28 days. After repeated applications, the half-life decreased to 14
days (Bailey and Coffey, 1985).
Carsel et al. (1986) studied the persistence of metalaxyl in various soil types. The
application rate was 2.2 kg/ha. In a fine sand, metalaxyl concentrations at soil depths of
15, 20, 45 and 60 cm were 100, 150, 100 and 75 ppb, respectively, 55 days after
Plant. In plants, metalaxyl undergoes ring oxidation, methyl ester hydrolysis, ether
cleavage, ring methyl hydroxylation and N-dealkylation (Owen and Donzel, 1986). Metalaxyl
acid was identified as a hydrolysis product in both sunflower leaves an
In pigeon peas, metalaxyl may persist up to 12 days (Indira et al., 1981; Chaube et
al., 1984).
Metabolic pathway
O-Demethylation is one of the major routes of metalaxyl degradation in the plant cell suspension culture. Although hydroxylation of methyl groups in the phenyl ring predominates in both lettuce and grapes, species differences are evident in grapes, whereas N-dealkylation and aryl hydroxylation are less important in lettuce. Two isomeric metabolites of methyl hydroxylation and the hydroxylated metabolite of the phenyl ring are identified as fungus metabolites. By UV irradiation of metalaxyl in aqueous solution, two rearrangement products of the N-acyl group to the 4-position on the phenyl ring are identified.
Shipping
UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.
Incompatibilities
Incompatible with alkaline materials, strong acids, oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Compounds of the carboxyl group react with all bases, both inorganic and organic (i.e., amines) releasing substantial heat, water and a salt that may be harmful. Incompatible with arsenic compounds (releases hydrogen cyanide gas), diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides (releasing heat, toxic, and possibly flammable gases), thiosulfates and dithionites (releasing hydrogen sulfate and oxides of sulfur).
Waste Disposal
Small amounts may be destroyed by alkaline hydrolysis. Admixture with alkali can be followed by soil burial. Larger quantities can be disposed of by incineration in admixture with acetone or xylene and using effluent gas scrubbing. Do not reuse empty container; proper disposal required.
Metalaxyl Preparation Products And Raw materials
Raw materials
1of4
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Indofil Industries Limited | +91-2266637373 +91-2266637373 | Maharashtra, India | 16 | 58 | Inquiry |
| Astec Lifesciences Ltd | +91-9870601018 +91-9870601013 | Maharashtra, India | 6 | 58 | Inquiry |
| OCEAN TRADING CORPORATION | +91(22) 24921669 | New Delhi, India | 6211 | 58 | Inquiry |
| Geekay Agriuno | 08068441051Ext 463 | Haryana, India | 3 | 58 | Inquiry |
| Kalyani Industries Limited | 08047639232 | Mumbai, India | 37 | 58 | Inquiry |
| Punjab Chemicals & Crop Protection Limited | +91 (22) 2674-7900 | New Delhi, India | 125 | 0 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Qingdao Trust Agri Chemical Co.,Ltd | +8613573296305 | China | 259 | 58 | Inquiry |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | China | 2503 | 58 | Inquiry |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | China | 21675 | 55 | Inquiry |
57837-19-1(Metalaxyl)Related Search:
1of4
chevron_right




