potassium trifluoromethanesulfonate
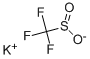
- CAS No.
- 41804-89-1
- Chemical Name:
- potassium trifluoromethanesulfonate
- Synonyms
- POTASSIUM TRIFLATE;Potassium triflinate;POTASSIUM TRIFLUOROMETHANESULFINATE;potassium trifluoromethanesulphinate;POTASSIUM TRIFLUOROMETHANESULPHONATE;POTASSIUM TRIFLUOROMETHANESULFONIC ACID;TRIFLUOROMETHANESULFONIC ACID POTASSIUM SALT;TRIFLUOROMETHANESULFINIC ACID, POTASSIUM SALT;Trifluoromethane sulphinic acid potassium salt;Methanesulfinic acid,1,1,1-trifluoro-, potassium salt (1:1)
- CBNumber:
- CB5687198
- Molecular Formula:
- CF3O2S.K
- Molecular Weight:
- 172.17
- MOL File:
- 41804-89-1.mol
- Modify Date:
- 2024/3/13 10:27:56
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H315-H319-H335 |
| Precautionary statements | P271-P261-P280 |
| Hazard Codes | Xi |
| Risk Statements | 36/37/38-38-37-36 |
| Safety Statements | 26-36 |
| WGK Germany | 3 |
| F | 3-10 |
| HS Code | 2930200090 |
potassium trifluoromethanesulfonate Chemical Properties,Uses,Production
Chemical Properties
white powder
Uses
Potassium Trifluoromethanesulfinate is used in preparation of trifluoromethanesulfonyl compounds.
Application
Potassium trifluoromethanesulfonate is used as a reagent in the synthesis of guanine-quadruplex hybrid materials.
Synthesis
At first, an aqueous solution was prepared by dissolving 125 g of potassium sulfite in 290 g of water. Then, a metal pressure-proof reactor was charged with this aqueous solution. Then, the atmosphere of the reactor was removed to obtain a reduced pressure. After that, 30.7 g of trifluoromethanesulfonic fluoride was added to the reactor, stirring from 0° C. to room temperature for 12 hr. The resulting reaction liquid was neutralized with potassium carbonate, followed by water removal. Then, methanol was added to the remaining solid matter to extract the target product. The resulting methanol solution was concentrated to dryness, obtaining 32.6 g of potassium trifluoromethanesulfonate (94%).
potassium trifluoromethanesulfonate Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6100 | 58 | Inquiry |
| JIANGXI TIME PHARMACEUTICAL CO.,LTD | +86-0794-7183888 +86-13155888520 | China | 27 | 58 | Inquiry |
| Fluoropharm Co., Ltd. | +86-0571-85586753 +86-13336034509 | China | 1445 | 60 | Inquiry |
| Shenzhen Nexconn Pharmatechs Ltd | +86-755-89396905 +86-15013857715 | China | 10406 | 58 | Inquiry |
| SHANGHAI T&W PHARMACEUTICAL CO., LTD. | +86-021-61551413 +8618813727289 | China | 5738 | 58 | Inquiry |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418671 +8618949823763 | China | 34563 | 58 | Inquiry |
| Allfluoro pharmaceutical co. ltd. | +86-400-0216110 +86-15958047586 | China | 11385 | 58 | Inquiry |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | China | 39894 | 58 | Inquiry |
| Shanghai Sunway Pharmaceutical Technology Co.,Ltd. | 021-51816796-820 13611835272 | China | 44455 | 58 | Inquiry |
| T&W GROUP | 021-61551611 13296011611 | China | 9895 | 58 | Inquiry |





