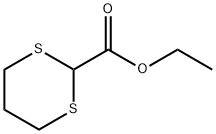
Ethyl 1,3-dithiane-2-carboxylate
- CAS No.
- 20462-00-4
- Chemical Name:
- Ethyl 1,3-dithiane-2-carboxylate
- Synonyms
- Carboethoxy-1,3-dithiane;2-Carboethoxy-1,3-dithiane;2-Ethoxycarbonyl-1,3-dithiane;ETHYL 1,3-DITHIANE-2-CARBOXYLATE;Ethyl1,3-Dithiane-2-carboxylate>Ethyl 1,3-dithiane-2-carboxylate, 98+%;ETHYL-1 3-DITHIANE-2-CARBOXYLATE 99% (&;ETHYL 1,3-DITHIANE-2-CARBOXYLATE FOR SYN;2-(2-phenylimino-3-thiazolidinyl)ethanol;m-Dithiane-2-carboxylic acid, ethyl ester
- CBNumber:
- CB6490333
- Molecular Formula:
- C7H12O2S2
- Molecular Weight:
- 192.3
- MOL File:
- 20462-00-4.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/10/31 10:56:06
| Melting point | 19-21°C |
|---|---|
| Boiling point | 75-77 °C/0.2 mmHg (lit.) |
| Density | 1.22 g/mL at 25 °C (lit.) |
| refractive index |
n |
| Flash point | 130 °F |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| form | clear liquid |
| color | Colorless to Light yellow to Light orange |
| Water Solubility | Slightly miscible with water. |
| BRN | 1424352 |
| InChI | InChI=1S/C7H12O2S2/c1-2-9-6(8)7-10-4-3-5-11-7/h7H,2-5H2,1H3 |
| InChIKey | ANEDZEVDORCLPM-UHFFFAOYSA-N |
| SMILES | S1CCCSC1C(OCC)=O |
| CAS DataBase Reference | 20462-00-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Ethyl 1,3-dithiane-2-carboxylate(20462-00-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS02 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H226 | |||||||||
| Precautionary statements | P241-P280a-P501a-P210-P233-P240-P241+P242+P243-P280-P303+P361+P353-P403+P235-P501 | |||||||||
| Risk Statements | 10 | |||||||||
| Safety Statements | 26-36/37/39-45 | |||||||||
| RIDADR | UN 3272 3/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| F | 9-13 | |||||||||
| HazardClass | 3 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29349990 | |||||||||
| NFPA 704 |
|
Ethyl 1,3-dithiane-2-carboxylate price More Price(5)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 43749 | Ethyl 1,3-dithiane-2-carboxylate technical, ≥90% (GC) | 20462-00-4 | 5ML | ₹14710 | 2022-06-14 | Buy |
| TCI Chemicals (India) | D1648 | Ethyl 1,3-Dithiane-2-carboxylate min. 98.0 % | 20462-00-4 | 1G | ₹2300 | 2022-05-26 | Buy |
| ALFA India | ALF-L00663-14 | Ethyl 1,3-dithiane-2-carboxylate, 98+% | 20462-00-4 | 25g | ₹32253 | 2022-05-26 | Buy |
| TCI Chemicals (India) | D1648 | Ethyl 1,3-Dithiane-2-carboxylate min. 98.0 % | 20462-00-4 | 5G | ₹8800 | 2022-05-26 | Buy |
| ALFA India | ALF-L00663-06 | Ethyl 1,3-dithiane-2-carboxylate, 98+% | 20462-00-4 | 5g | ₹5805 | 2022-05-26 | Buy |
Ethyl 1,3-dithiane-2-carboxylate Chemical Properties,Uses,Production
Chemical Properties
clear yellowish liquid
Uses
Ethyl 1,3-dithiane-2-carboxylate is used in syn-selective aldol reactions. It undergoes asymmetric oxidation to give trans bis-sulfoxide. It is used to generate carbanon, which finds application in the preparation of alfa- keto esters.
Preparation
To a solution of BF3. Et2O (57.5 mL, 0.454 mmol) in CHC13 (180 mL) heated at reflux was added dropwise, over a 1h period, a solution of 1,3-propanedithiol (22.7 mL, 0.227 mmol), followed by ethyl diethoxyacetate (40 g, 0.227 mmol) in CHCl3 (40 mL). The resulting mixture was heated for 30 minutes, and then cooled to rt. The cooled solution was washed 2 times with water, once with saturated aqueous NaHC03, and then re-washed with water. The combined organic phases were dried over MgS04, then evaporated to give 41 g (94%) of Ethyl 1,3-dithiane-2-carboxylate as a yellow oi. Yield: 94%
Application
Ethyl 1,3-dithiane-2-carboxylate is a synthetic compound that is used in the synthesis of organic compounds . It is used in asymmetric synthesis to produce chiral products from achiral starting materials. The desulfurization reaction of ethyl 1,3-dithiane-2-carboxylate produces a mixture of the two possible stereoisomers of the product. This mixture can be separated by phase chromatography and then hydrolyzed to give the desired product. In dyslipidemia, ethyl 1,3-dithiane-2-carboxylate inhibits the activity of hepatic lipase and acidolysis. This inhibition leads to an increase in serum triglycerides and cholesterol levels. This compound also has been shown to have antiplatelet properties when administered orally or intravenously to rats without affecting platelet aggregation rates.
General Description
Ethyl 1,3-dithiane-2-carboxylate is an α-keto acid equivalent and bulky equivalent of acetate. It participates in syn-selective aldol reactions. It can be prepared from the reaction of ethyl diethoxyacetate and 1,3-propanedithiol in the presence of BF3/Et2O. Asymmetric oxidation of ethyl 1,3-dithiane-2-carboxylate by Modena protocol has been reported to afford trans bis-sulfoxide in 60% yield. Carbanion from ethyl 1,3-dithiane-2-carboxylate may be employed for the preparation of α-keto esters.
Purification Methods
Dissolve the ester in CHCl3, wash with aqueous K2CO3, twice with H2O, dry over MgSO4, filter, evaporate and distil the residue. [Eliel & Hartman J Org Chem 37 505 1972, Seebach Synthesis 1 17 1969, Beilstein 19/7 V 227.]
Ethyl 1,3-dithiane-2-carboxylate Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CHEMSWORTH | +91-261-2397244 | New Delhi, India | 6707 | 30 | Inquiry |
| TCI Chemicals (India) Pvt. Ltd. | 1800 425 7889 | New Delhi, India | 6778 | 58 | Inquiry |
| Alfa Aesar | 1 800 209 7001 | Maharashtra, India | 6913 | 58 | Inquiry |
| Huida Pharmaceutical Technology (Shanghai) Co., Ltd. | +8613601750004 | China | 439 | 58 | Inquiry |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | China | 29798 | 60 | Inquiry |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | China | 16220 | 58 | Inquiry |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | China | 32760 | 60 | Inquiry |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | China | 49391 | 58 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29826 | 58 | Inquiry |
| Neostar United (Changzhou) Industrial Co., Ltd. | +86-519-519-85557386 | China | 12210 | 58 | Inquiry |
20462-00-4(Ethyl 1,3-dithiane-2-carboxylate)Related Search:
1of4
chevron_right




