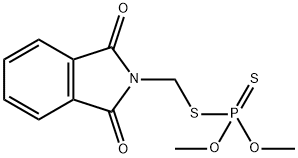
Phosmet
- CAS No.
- 732-11-6
- Chemical Name:
- Phosmet
- Synonyms
- PMP;IMIDN;imidan;INOVAT;FOSDAN;Fosmet;R-1504;Smidan;CEKUMET;PHOSMET
- CBNumber:
- CB6681168
- Molecular Formula:
- C11H12NO4PS2
- Molecular Weight:
- 317.32
- MOL File:
- 732-11-6.mol
- Modify Date:
- 2025/1/27 9:38:02
| Melting point | 72.5°C |
|---|---|
| Boiling point | 412.6±47.0 °C(Predicted) |
| Density | 1.03 g/cm3 |
| vapor pressure | 6.5×10-5 Pa (25°C) |
| Flash point | >100 °C |
| storage temp. | 0-6°C |
| solubility | Chloroform: Slightly Soluble,Methanol: Slightly Soluble |
| Water Solubility | 25 mg l-1 (25°C) |
| pka | -2.63±0.20(Predicted) |
| form | solid |
| BRN | 264869 |
| LogP | 2.780 |
| CAS DataBase Reference | 732-11-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Phosmet(732-11-6) |
| EPA Substance Registry System | Phosmet (732-11-6) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS06,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301-H332-H361f-H370-H410 | |||||||||
| Precautionary statements | P202-P260-P264-P273-P301+P310-P304+P340+P312 | |||||||||
| Hazard Codes | Xn;N,N,Xn | |||||||||
| Risk Statements | 21/22-50/53 | |||||||||
| Safety Statements | 22-36/37-60-61 | |||||||||
| RIDADR | UN 2811 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | TE2275000 | |||||||||
| HazardClass | 6.1(b) | |||||||||
| PackingGroup | III | |||||||||
| Hazardous Substances Data | 732-11-6(Hazardous Substances Data) | |||||||||
| Toxicity | LD50 in male, female rats (mg/kg): 113, 160 orally (Gaines) | |||||||||
| NFPA 704 |
|
Phosmet price More Price(2)
Phosmet Chemical Properties,Uses,Production
Description
Phosmet is an organophosphate insecticide and acaricide. It reduces apple damage by a large variety of insects, including apple maggots, codling moths, and obliquebanded leafrollers when used as either a border or cover spray at a concentration of 1.9 kg AI/hectare. Phosmet is effective in controlling S. scabiei in pigs when applied as a 20% pour-on solution. It is toxic to rats via oral administration (LC50 = 230 mg/kg). Formulations containing phosmet have been used in the control of insects and mites in agriculture.
Chemical Properties
Phosmet is a white crystalline solid
Uses
Phosmet is used as a pesticide to protect crops and vegetation. Insecticide.
General Description
Off-white crystalline solid with an offensive odor. Used as an insecticide and acaricide.
Reactivity Profile
Organophosphates, such as Phosmet, are susceptible to formation of highly toxic and flammable phosphine gas in the presence of strong reducing agents such as hydrides. Partial oxidation by oxidizing agents may result in the release of toxic phosphorus oxides. Storage above 113 F, may lead to decomposition. [EPA, 1998].
Health Hazard
Phosmet is an organophosphorus pesticide. Phosmet is very toxic; the probable oral lethal dose for humans is 50-500 mg/kg, or between 1 teaspoon and 1 oz. for a 150 lb. person. It is a cholinesterase inhibitor and has central nervous system effects. Oral lethal doses in humans have been reported at 50 mg/kg.
Fire Hazard
(Non-Specific -- Organophosphorus Pesticide, n.o.s.). Container may explode in heat of fire. Fire and runoff from fire control water may produce irritating or poisonous gases. Storage above 113F, may lead to decomposition.
Agricultural Uses
Insecticide, Acaricide: Phosmet is a non-systemic insecticide used on both plants and animals. It is mainly used on apple trees for control of coddling moth, though it is also used on a wide range of crops including alfalfa, nuts, grapes, blueberries, peas, potatoes, fruit crops, ornamentals, and vines for the control of aphids, suckers, mites, fire ants and fruit flies. The compound is also an active ingredient in some dog collars. It is used as an insecticide on swine and cattle. U.S. Maximum Allowable Residue Levels for Phosmet [40 CFR 180.261 (a)]: Alfalfa 40 ppm; almond, hulls 10 ppm; apple 10 ppm; apricot 5 ppm; blueberry 10 ppm; cattle, fat 0.2 ppm; cattle, meat 0.2 ppm; cattle, meat byproducts 0.2 ppm; cherry 10 ppm; cotton, undelinted seed 0.1 ppm; crabapple 20 ppm; cranberry 10 ppm; fruit, stone, group 12 5 ppm; goat, fat 0.2 ppm; goat, meat0.2 ppm; goat, meat byproducts 0.2 ppm; grape 10 ppm; hog, fat 0.2 ppm; hog, meat 0.2 ppm; hog, meat byproducts 0.2 ppm; horse, fat 0.2 ppm; horse, meat 0.2 ppm; horse, meat byproducts 0.2 ppm; kiwifruit 25 ppm; nectarine 5 ppm; nuts 0.1 ppm; pea 0.5 ppm; pea, field, hay 10 ppm; pea, field, vines 10 ppm; peach 10 ppm; pear 10 ppm; pistachio 0.1 ppm; plum, prune, fresh 5 ppm; potato 0.1 ppm; sheep, fat 0.2 ppm; sheep, meat 0.2 ppm; sheep, meat byproducts 0.2 ppm; sweet potato, roots 10 ppm. Regional registration, as defined in section 180.1(n) [40 CFR 180.261 (c)]: Crabapple 20 ppm; pistachio 0.1 ppm.
Trade name
Trade Names: APPA®; DECEMTHION®; DELPHOS ®; FESDAN®; FIREBAN®; FTALOPHOS®; IMIDAN®; KEMOLATE®; PERCOLATE®; PMC®; PROLATE®[C]; R 1504®; SAFIDON®; SMIDAN®; STARBAR CATTLE DUST®[C]; STAUFFER R 1504®; VET-KEM®; ZEOCON®
Safety Profile
A human poison by ingestion. Poison experimentally by inhalation and ingestion routes. Moderately toxic by skin contact. Human systemic effects by inhalation: lachqmation, somnolence, and olfaction effects. Experimental teratogenic and reproductive effects. Mutation data reported. When heated to decomposition it emits very toxic fumes of NOx, POx, and SOx. See also ESTERS.
Potential Exposure
An organophosphorus insecticide and acaricide.
Carcinogenicity
No treatment-related increases
in tumor incidence occurred in rats given a diet with 0, 20, 40,200, or 400 ppm (400 ppm terminated at 12 months) (equivalent
to 0, 1.1, 1.8, 9.4, and 23 mg/kg/day in males and 0, 1.1,
2.1, 10.9, and 27 mg/kg/day in females) for 2 years .
When mice were given diets with 0, 5, 25, or 100 ppm
phosmet (approximately 0.75, 3.75, or 15.0 mg/kg/day) for
2 years, there was an increased incidence of hepatocellular
tumors in males and mammary gland tumors in
females .
Environmental Fate
Soil. In soils, phosmet is rapidly hydrolyzed to phthalimide (Camazano and Martin,
1980; Sánchez Camazano and Sánchez Martin, 1983). The rate of hydrolysis is greater in
the presence of various montmorillonite clays and chloride salts. The calculated hydrolysis
half-lives of phosmet in the presence of calcium, barium, copper, magnesium and nickel
montmorillonite clays were 0.084, 0.665, 10.025, 16.926 and 28.738 days, respectively.
Similarly, the half-lives of phosmet in the presence of copper, calcium, magnesium and
barium chlorides were <0.020, 5.731, 10.680 and 12.242 days, respectively. In comparison,
the hydrolysis of phosmet in a neutral water solution was 46.210 days (Sánchez Camazano
and Sánchez Martin, 1983)
Plant. In plants, phosmet is degraded to nontoxic compounds (Hartley and Kidd, 1987).
Dorough et al. (1966) reported the half-life in Bermuda grass was 6.5 days. Half-life values
ranged from 1.2 to 6.5 days (Dorough et al., 1966; Leuck and Bowman
Chemical/Physical. Emits toxic fumes of phosphorus, nitrogen and sulfur oxides when
heated to decomposition (Sax and Lewis, 1987). Though no products were identified, the
hydrolysis half-lives at 20°C were 7.0 days and 7.1 hours at pH 6.1 and pH 7.4, respectively.
At 37.5°C and pH 7.4, the hydrolysis is 1.1 hours (Freed et al., 1979)
Metabolic pathway
The main degradative route of phosmet metabolism in plants and animals is similar with the compound being hydrolysed via attack on the carbonyl group of the phthaloyl moiety, yielding phthalamic acid which is further hydrolysed and decarboxylated to benzoic acid. It should be noted, however, that the studies on which the evidence for these metabolites is based are old and used paper chromatography for the main analytical evidence. A rather later study in rats identified N-hydroxymethylphthalimide and phthalimide as metabolites, implying a degradative hydrolytic route via attack on the N-methylene carbon atom. Oxidative desulfuration to the active acetylcholinesterase inhibitor phosmet oxon predominates over hydrolysis in insects and is responsible for the selectivity of the compound.
Metabolism
Orally administered phosmet in mammals is rapidly degraded to phthalamic acid, phthalic acid, and phthalic acid derivatives, and the metabolites are excreted in the urine. Oxidative desulfuration to the oxon predominates over hydrolysis in insects. It is rapidly degraded in plants and soils.
Shipping
UN2783 Organophosphorus pesticides, solid, toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials. UN3018 Organophosphorus pesticides, liquid, toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials. UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1Poisonous materials, Technical Name Required.
Incompatibilities
Organothiophosphates are susceptible to formation of highly toxic and flammable phosphine gas in the presence of strong reducing agents such as hydrideds and active metals. Partial oxidation by oxidizing agents may result in the release of toxic phosphorus oxides. Compounds of the carboxyl group react with all bases, both inorganic and organic (i.e., amines) releasing substantial heat, water and a salt that may be harmful. Incompatible with arsenic compounds (releases hydrogen cyanide gas), diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides (releasing heat, toxic and possibly flammable gases), thiosulfates and dithionites (releasing hydrogen sulfate and oxides of sulfur). Not compatible with other pesticides under alkaline conditions. Contact with water, steam or moisture forms phthalic acids. Slightly corrosive to metals in the presence of moisture.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office. Small amounts may be decomposed with hypochlorite. For large amounts, incineration with effective gas scrubbing is recommended.
Phosmet Preparation Products And Raw materials
Raw materials
1of3
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6257 | 58 | Inquiry |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | China | 39894 | 58 | Inquiry |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | China | 49732 | 58 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29808 | 58 | Inquiry |
| Hebei Chuanghai Biotechnology Co., Ltd | +8615531151365 | China | 18137 | 58 | Inquiry |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 | China | 49984 | 58 | Inquiry |
| SUZHOU SENFEIDA CHEMICAL CO.,LTD | +86-0512-83500002 +8615195660023 | China | 26362 | 58 | Inquiry |
| Aladdin Scientific | United States | 57505 | 58 | Inquiry | |
| XIAMEN AMITY INDUSTRY AND TRADE CO., LTD. | +8618950047208 | China | 43416 | 58 | Inquiry |
| LGC Standards | +44 (0)20 8943 8480 | United Kingdom | 6494 | 0 | Inquiry |
732-11-6(Phosmet)Related Search:
1of4
chevron_right




