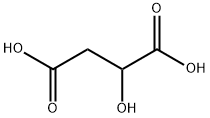
Malic acid
- CAS No.
- 6915-15-7
- Chemical Name:
- Malic acid
- Synonyms
- malic;DL-Malic;DL-MALATE;hydroxy-butanedioicaci;DL-MALIC ACID FREE ACID;alpha-Hydroxysuccinic acid;-MaL;E 296;FDA 2018;malicaci
- CBNumber:
- CB7220795
- Molecular Formula:
- C4H6O5
- Molecular Weight:
- 134.09
- MOL File:
- 6915-15-7.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/8/7 19:09:42
| Melting point | 131-133 °C (lit.) |
|---|---|
| Boiling point | 167.16°C (rough estimate) |
| alpha | [α]D20 -0.5~+0.5° (c=5, H2O) |
| Density | 1.609 |
| vapor density | 4.6 (vs air) |
| vapor pressure | <0.1 mm Hg ( 20 °C) |
| refractive index | 1.3920 (estimate) |
| Flash point | 203 °C |
| storage temp. | 2-8°C |
| solubility | methanol: 0.1 g/mL, clear, colorless |
| pka | 3.4(at 25℃) |
| form | Solid |
| color | White to Almost white |
| Odor | at 100.00 %. odorless |
| PH | 3.33(1 mM solution);2.74(10 mM solution);2.21(100 mM solution); |
| optical activity | [α]/D 0.10 to +0.10° |
| Odor Type | odorless |
| Water Solubility | 558 g/L (20 ºC) |
| Merck | 14,5707 |
| BRN | 1723539 |
| LogP | -6.14 at 20℃ |
| CAS DataBase Reference | 6915-15-7(CAS DataBase Reference) |
| NIST Chemistry Reference | Hydroxybutanedioic acid(6915-15-7) |
| EPA Substance Registry System | Malic acid (6915-15-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H319 | |||||||||
| Precautionary statements | P264-P280-P305+P351+P338-P337+P313 | |||||||||
| Hazard Codes | Xn,Xi | |||||||||
| Risk Statements | 22-37/38-41-36/37/38-42/43-34 | |||||||||
| Safety Statements | 26-39-37/39-36-36/39 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | ON7175000 | |||||||||
| Autoignition Temperature | 644 °F | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 29181980 | |||||||||
| Toxicity | LD50 orally in Rabbit: > 3200 mg/kg | |||||||||
| NFPA 704 |
|
Malic acid price More Price(25)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | W265501 | DL-Malic acid 99% | 6915-15-7 | 1SAMPLE-K | ₹5141.88 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | W265501 | DL-Malic acid 99% | 6915-15-7 | 1KG | ₹7220.28 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | W265501 | DL-Malic acid 99% | 6915-15-7 | 5KG | ₹11864.2 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | W265501 | DL-Malic acid 99% | 6915-15-7 | 10KG | ₹17287.53 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | PHR1273 | Malic acid Pharmaceutical Secondary Standard; Certified Reference Material | 6915-15-7 | 1G | ₹8107.93 | 2022-06-14 | Buy |
Malic acid Chemical Properties,Uses,Production
Uses
Malic acid, HOOCCH(OH).CH2COOH, also known as hydroxysuccinic acid, is a colorless solid. It is soluble in water and alcohol. Malic acid exists in two optically active forms and a racemic mixture. It is used in medicine and found in apples and other fruits.
The naturally occuring isomer is the L-form which has been found in apples and many other fruits and plants. Selective α-amino protecting reagent for amino acid derivatives. Versatile synthon for the preparation of chiral compounds including κ-opioid rece
Production Methods
Malic acid is manufactured by hydrating maleic and fumaric acids in the presence of suitable catalysts. The malic acid formed is then separated from the equilibrium product mixture.
Definition
ChEBI: Malic acid is a 2-hydroxydicarboxylic acid that is succinic acid in which one of the hydrogens attached to a carbon is replaced by a hydroxy group. It has a role as a food acidity regulator and a fundamental metabolite. It is a 2-hydroxydicarboxylic acid and a C4-dicarboxylic acid. It derives from a succinic acid. It is a conjugate acid of a malate(2-) and a malate.
General Description
The chiral resolution of DL-malic acid by ligand-exchange capillary electrophoresis was studied.
Pharmaceutical Applications
Malic acid is used in pharmaceutical formulations as a generalpurpose
acidulant. It possesses a slight apple flavor and is used as a
flavoring agent to mask bitter tastes and provide tartness. Malic
acid is also used as an alternative to citric acid in effervescent
powders, mouthwashes, and tooth-cleaning tablets.
In addition, malic acid has chelating and antioxidant properties.
It may be used with butylated hydroxytoluene as a synergist in order
to retard oxidation in vegetable oils. In food products it may be used
in concentrations up to 420 ppm.
Therapeutically, malic acid has been used topically in combination
with benzoic acid and salicylic acid to treat burns, ulcers, and
wounds. It has also been used orally and parenterally, either
intravenously or intramuscularly, in the treatment of liver disorders,
and as a sialagogue.
Mechanism of action
Malic acid is absorbed from the gastrointestinal tract from whence it is transported via the portal circulation to the liver. There are a few enzymes that metabolize malic acid. Malic enzyme catalyzes the oxidative decarboxylation of L-malate to pyruvate with concomitant reduction of the cofactor NAD+ (oxidized form of nicotinamide adenine dinucleotide) or NADP+ (oxidized form of nicotinamide adenine dinucleotide phosphate). These reactions require the divalent cations magnesium or manganese. Three isoforms of malic enzyme have been identified in mammals: a cytosolic NADP+-dependent malic enzyme, a mitochondrial NADP+- dependent malic enzyme and a mitochondrial NAD(P)+-dependent malic enzyme. The latter can use either NAD+ or NADP+ as the cofactor but prefers NAD+. Pyruvate formed from malate can itself be metabolized in a number of ways, including metabolism via a number of metabolic steps to glucose. Malate can also be metabolized to oxaloacetate via the citric acid cycle. The mitochondrial malic enzyme, particularly in brain cells may play a key role in the pyruvate recycling pathway, which utilizes dicarboxylic acids and substrates, such as glutamine, to provide pyruvate to maintain the citric acid cycle activity when glucose and lactate are low.
Safety Profile
A poison by intraperitoneal route. Moderately toxic by ingestion. A skin and severe eye irritant. When heated to decomposition it emits acrid smoke and irritating fumes.
Safety
Malic acid is used in oral, topical, and parenteral pharmaceutical
formulations in addition to food products, and is generally regarded
as a relatively nontoxic and nonirritant material. However,
concentrated solutions may be irritant.
LD50 (rat, oral): 1.6 g/kg(3)
LD50 (rat, IP): 0.1 g/kg
storage
Malic acid is stable at temperatures up to 150°C. At temperatures
above 150°C it begins to lose water very slowly to yield fumaric
acid; complete decomposition occurs at about 180°C to give
fumaric acid and maleic anhydride.
Malic acid is readily degraded by many aerobic and anaerobic
microorganisms. Conditions of high humidity and elevated
temperatures should be avoided to prevent caking.
The effects of grinding and humidity on malic acid have also
been investigated.
The bulk material should be stored in a well-closed container, in
a cool, dry place.
Purification Methods
Crystallise the acid from acetone, then from acetone/CCl4, or from ethyl acetate by adding pet ether (b 60-70o). Dry it at 35o under 1mm pressure to avoid formation of the anhydride. [Beilstein 3 IV 1124.]
Incompatibilities
Malic acid can react with oxidizing materials. Aqueous solutions are mildly corrosive to carbon steels.
Regulatory Status
GRAS listed. Both the racemic mixture and the levorotatory isomer are accepted as food additives in Europe. The DL and L forms are included in the FDA Inactive Ingredients Database (oral preparations). Included in nonparenteral and parenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Nonmedicinal Ingredients.
Malic acid Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| ANJI BIOSCIENCES | +91-9000100077 +91-9000100077 | Hyderabad, India | 430 | 58 | Inquiry |
| JSK Chemicals | +919879767970 | Gujarat, India | 3756 | 58 | Inquiry |
| Merck Ltd | +91-2262109800 +91-2262109000 | Maharashtra, India | 272 | 58 | Inquiry |
| Nandu Chemical Industries | +91-8362330469 +91-8362330469 | Karnataka, India | 84 | 58 | Inquiry |
| Prakash Chemicals Agencies | +91-8511140657 | Gujarat, India | 106 | 58 | Inquiry |
| Thirumalai Chemicals Ltd | +91-2224017841 +91-9892152216 | Maharashtra, India | 4 | 58 | Inquiry |
| ASM Organics | 91-9866122393 | Andhra Pradesh, India | 1188 | 58 | Inquiry |
| Anmol Chemicals (Mubychem Group Company) | 91-22-23770100 | Maharashtra, India | 63 | 58 | Inquiry |
| Pharma Affiliates | 172-5066494 | Haryana, India | 6761 | 58 | Inquiry |
| B. I. Mehta | 08048989571 | Mumbai, India | 26 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| ANJI BIOSCIENCES | 58 |
| JSK Chemicals | 58 |
| Merck Ltd | 58 |
| Nandu Chemical Industries | 58 |
| Prakash Chemicals Agencies | 58 |
| Thirumalai Chemicals Ltd | 58 |
| ASM Organics | 58 |
| Anmol Chemicals (Mubychem Group Company) | 58 |
| Pharma Affiliates | 58 |
| B. I. Mehta | 58 |
Related articles
- Malic acid: Significance and its Quantifying Methods
- Malic acid, pivotal in food and industry, demands monitoring for quality. Biosensors, especially electrochemical, offer effici....
- May 14,2024
- Why is Malic acid used in food
- Malic acid is used to increase food acidity, giving more flavor, but it is also used as a flavoring substance and color stabil....
- May 13,2024
- Malic Acid: Preparation and Applications
- Malic acid is used primarily as an ingredient in hard candys and other sweets, jams, jellies, and various canned fruits and ve....
- Oct 21,2023
6915-15-7(Malic acid )Related Search:
1of4
chevron_right




