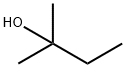
2-Methyl-2-butanol
- CAS No.
- 75-85-4
- Chemical Name:
- 2-Methyl-2-butanol
- Synonyms
- TERT-AMYL ALCOHOL;T-AMYL ALCOHOL;2-METHYLBUTAN-2-OL;t-amyl;TERT-PENTANOL;AMYLENE HYDRATE;2-methyl-2-butano;TERT-PENTYL ALCOHOL;tert-Isoamyl alcohol;Tert-AmylAlcohol,99%
- CBNumber:
- CB7854246
- Molecular Formula:
- C5H12O
- Molecular Weight:
- 88.15
- MOL File:
- 75-85-4.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/7/30 16:49:16
| Melting point | -12 °C |
|---|---|
| Boiling point | 102 °C(lit.) |
| Density | 0.805 g/mL at 25 °C(lit.) |
| vapor density | 3 (vs air) |
| vapor pressure | 15.5 hPa (20 °C) |
| refractive index |
n |
| Flash point | 20 °C |
| storage temp. | Store at +5°C to +30°C. |
| solubility | Miscible with alcohol, ether, benzene, chloroform, glycerol, oils and acetone. |
| form | Liquid |
| pka | 15.38±0.29(Predicted) |
| color | Clear colorless |
| PH | 6.0 (118g/l, H2O, 20℃)neutral |
| Odor | pungent |
| explosive limit | 1.3-9.6%(V) |
| Odor Threshold | 0.088ppm |
| Water Solubility | 120 g/L (20 ºC) |
| Merck | 14,7140 |
| BRN | 1361351 |
| Dielectric constant | 11.7(20℃) |
| Stability | Light sensitive. Highly flammable. Incompatible with strong oxidizing agents. |
| LogP | 0.890 |
| CAS DataBase Reference | 75-85-4(CAS DataBase Reference) |
| NIST Chemistry Reference | 2-Butanol, 2-methyl-(75-85-4) |
| EPA Substance Registry System | 2-Methyl-2-butanol (75-85-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS02,GHS05,GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H225-H312+H332-H315-H318-H335 | |||||||||
| Precautionary statements | P210-P233-P280-P303+P361+P353-P304+P340+P312-P305+P351+P338 | |||||||||
| Hazard Codes | F,Xn | |||||||||
| Risk Statements | 11-20-37/38-41-21 | |||||||||
| Safety Statements | 46-39-36/37-26 | |||||||||
| RIDADR | UN 1105 3/PG 2 | |||||||||
| OEB | A | |||||||||
| OEL | TWA: 100.0 ppm; 360.0 mg/m3, STEL: 125.0 ppm; 450.0 mg/m3 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | SC0175000 | |||||||||
| Autoignition Temperature | 819 °F | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 3 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 29051500 | |||||||||
| Toxicity | LD50 orally in rats: 1.0 g/kg (Schaffarzick, Brown) | |||||||||
| NFPA 704 |
|
2-Methyl-2-butanol price More Price(19)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | PHR1667 | t-Amyl Alcohol Pharmaceutical Secondary Standard; Certified Reference Material | 75-85-4 | 3X1.2ML | ₹9103.83 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 721123 | 2-Methyl-2-butanol anhydrous, ≥99% | 75-85-4 | 100ML | ₹9298.68 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 8.06193 | tert-Amyl alcohol EMPLURA? | 75-85-4 | 1L | ₹7040 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 721123 | 2-Methyl-2-butanol anhydrous, ≥99% | 75-85-4 | 1L | ₹12784.33 | 2022-06-14 | Buy |
| Sigma-Aldrich | 240486 | 2-Methyl-2-butanol ReagentPlus?, ≥99% | 75-85-4 | 5ML | ₹3799.58 | 2022-06-14 | Buy |
2-Methyl-2-butanol Chemical Properties,Uses,Production
Chemical Properties
tert-Amyl alcohol is a volatile liquid.2-Methyl-2-butanol has a sour odor, a threshold value of 8.2 mg/m3 (2.3 ppm), an absolute perception limit of 0.04 ppm, and a 100% recognition level of 0.23 ppm .
Uses
2-Methyl-2-butanol is used as a solvent in flavors, pharmaceuticals, corrosion inhibitors, resins, gums, coating materials and in organic synthesis. It acts as a frothing agent (ore flotation), surfactant (petroleum recovery) and stabilizing agent. Further, it is used in processing aids.
Production Methods
tert-Amyl alcohol is prepared by hydrating 2-methyl-2- butenes. It can also be prepared by reducing pivalic acid.
Definition
ChEBI: A tertiary alcohol that is propan-1-ol in which both of the hydrogens at position 1 have been replaced by methyl groups.
General Description
A clear, colorless liquid with an odor of camphor. Flash point 70°F. Density 0.81 g / cm3. Slightly soluble in water.
Air & Water Reactions
Highly flammable. Slightly soluble in water.
Reactivity Profile
2-Methyl-2-butanol attacks plastics [Handling Chemicals Safely 1980. p. 236]. Reacts violently with acetyl bromide [Merck 11th ed. 1989]. Mixtures of alcohols with concentrated sulfuric acid and strong hydrogen peroxide can cause explosions. Example: an explosion will occur if dimethylbenzylcarbinol is added to 90% hydrogen peroxide then acidified with concentrated sulfuric acid. Mixtures of ethyl alcohol with concentrated hydrogen peroxide form powerful explosives. Mixtures of hydrogen peroxide and 1-phenyl-2-methyl propyl alcohol tend to explode if acidified with 70% sulfuric acid [Chem. Eng. News 45(43):73 1967; J, Org. Chem. 28:1893 1963]. Alkyl hypochlorites are violently explosive. They are readily obtained by reacting hypochlorous acid and alcohols either in aqueous solution or mixed aqueous-carbon tetrachloride solutions. Chlorine plus alcohols would similarly yield alkyl hypochlorites. They decompose in the cold and explode on exposure to sunlight or heat. Tertiary hypochlorites are less unstable than secondary or primary hypochlorites [NFPA 491 M, 1991]. Base-catalysed reactions of isocyanates with alcohols should be carried out in inert solvents. Such reactions in the absence of solvents often occur with explosive violence [Wischmeyer 1969].
Hazard
Flammable, dangerous fire risk.
Health Hazard
Inhalation or contact with material may irritate or burn skin and eyes. Fire may produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control may cause pollution.
Fire Hazard
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Safety Profile
Moderately toxic to humans by an unspecified route. Moderately toxic experimentally by ingestion, intraperitoneal, subcutaneous, and rectal routes. Narcotic in high concentration. Flammable liquid when exposed to heat, flame, or oxiduing materials. Moderately explosive in the form of vapor when exposed to heat or flame. A hypnotic agent. When heated to decomposition it emits acrid smoke and irritating fumes.
Potential Exposure
(n-isomer); Suspected reprotoxic hazard, Primary irritant (w/o allergic reaction), (iso-, primary): Possible risk of forming tumors, Primary irritant (w/o allergic reaction), (sec-, active primary-, and other isomers) Primary irritant (w/o allergic reaction). Used as a solvent in organic synthesis and synthetic flavoring, pharmaceuticals, corrosion inhibitors; making plastics and other chemicals; as a flotation agent. The (n-isomer) is used in preparation of oil additives, plasticizers, synthetic lubricants, and as a solvent.
Shipping
UN2811 Pentanols, Hazard Class: 3; Labels: 3- Flammable liquid. UN1987 Alcohols, n.o.s., Hazard Class: 3; Labels: 3-Flammable liquid.
Purification Methods
Reflux it with K2CO3, CaH2, CaO or sodium, then fractionally distil. The near-dry alcohol is further dried by refluxing with Mg activated with iodine, as described for ethanol. Further purification is possible using fractional crystallisation and zone refining at <-10o or preparative gas chromatography. [Beilstein 1 IV 1668.]
Incompatibilities
Forms an explosive mixture with air. Contact with strong oxidizers and hydrogen trisulfide may cause fire and explosions. Incompatible with strong acids. Violent reaction with alkaline earth metals forming hydrogen, a flammable gas.
Waste Disposal
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed.
Precautions
During handling and use of amyl alcohol, persons with pre-existing skin disorders, eye problems, or impaired liver, kidney, or respiratory function, should be careful since these workers/persons are more susceptible to the effects of amyl alcohol
2-Methyl-2-butanol Preparation Products And Raw materials
Raw materials
1of3
chevron_rightPreparation Products
1of3
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| ANJI BIOSCIENCES | +91-9000100077 +91-9000100077 | Hyderabad, India | 430 | 58 | Inquiry |
| BASF India Limited | +91-2262785600 +91-2262785600 | Maharashtra, India | 209 | 58 | Inquiry |
| BASF India Limited | 91-22-67127600 | Maharashtra, India | 138 | 58 | Inquiry |
| Loba Chemie Pvt., Ltd. | 91-22-66636663 | Maharashtra, India | 766 | 58 | Inquiry |
| Jay Chem Marketing | 08048964412 | Mumbai, India | 231 | 58 | Inquiry |
| Pacific Agencies | 08048372547Ext 801 | Mumbai, India | 135 | 58 | Inquiry |
| Innovative | 07942565925 | Mumbai, India | 617 | 58 | Inquiry |
| J. N. Chemical | 91-260-2435225 | Gujarat, India | 139 | 58 | Inquiry |
| Vijay Chemicals | 08048971555 | Pune, India | 50 | 58 | Inquiry |
| ALPHA CHEMIKA | +91-22-22061123 +91-22-66382501 | Mumbai, India | 1681 | 43 | Inquiry |
| Supplier | Advantage |
|---|---|
| ANJI BIOSCIENCES | 58 |
| BASF India Limited | 58 |
| BASF India Limited | 58 |
| Loba Chemie Pvt., Ltd. | 58 |
| Jay Chem Marketing | 58 |
| Pacific Agencies | 58 |
| Innovative | 58 |
| J. N. Chemical | 58 |
| Vijay Chemicals | 58 |
| ALPHA CHEMIKA | 43 |
Related articles
- tert-Pentanol: Properties, Process for manufacture and Uses
- tert-Pentanol is mainly used as a solvent and an intermediate for some pharmaceuticals and fine chemicals. It is used as an in....
- Mar 4,2024
75-85-4(2-Methyl-2-butanol)Related Search:
1of4
chevron_right




