Roxadustat
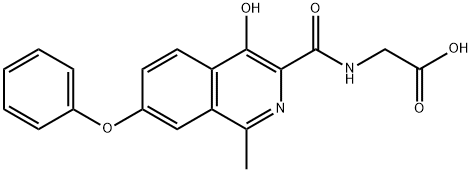
- CAS No.
- 808118-40-3
- Chemical Name:
- Roxadustat
- Synonyms
- FG-4592;Roxadustat (FG-4592);N-[(4-Hydroxy-1-Methyl-7-phenoxy-3-isoquinolinyl)carbonyl]glycine;2-(4-hydroxy-1-Methyl-7-phenoxyisoquinoline-3-carboxaMido)acetic acid;ASP1517;FG-4592 (Roxadustat);IBT4A;CS-185;Rosasta;FibroGen
- CBNumber:
- CB82538253
- Molecular Formula:
- C19H16N2O5
- Molecular Weight:
- 352.34
- MOL File:
- 808118-40-3.mol
- Modify Date:
- 2025/2/19 21:00:37
| Melting point | 199-215°C |
|---|---|
| Boiling point | 684.3±55.0 °C(Predicted) |
| Density | 1.389 |
| storage temp. | -20°C |
| solubility | Soluble in DMSO (up to at least 25 mg/ml). |
| form | solid |
| pka | 2.46±0.10(Predicted) |
| color | Off-white or white |
| Stability | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 2 months. |
| InChI | InChI=1S/C19H16N2O5/c1-11-15-9-13(26-12-5-3-2-4-6-12)7-8-14(15)18(24)17(21-11)19(25)20-10-16(22)23/h2-9,24H,10H2,1H3,(H,20,25)(H,22,23) |
| InChIKey | YOZBGTLTNGAVFU-UHFFFAOYSA-N |
| SMILES | C(O)(=O)CNC(C1=C(O)C2=C(C(C)=N1)C=C(OC1=CC=CC=C1)C=C2)=O |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H319-H302-H315-H332-H335 |
| Precautionary statements | P264-P280-P305+P351+P338-P337+P313P-P264-P280-P302+P352-P321-P332+P313-P362-P264-P270-P301+P312-P330-P501-P261-P271-P304+P340-P312 |
Roxadustat Chemical Properties,Uses,Production
Description
Roxadustat is an orally administered, small molecule hypoxia-inducible factor (HIF) prolyl hydroxylase inhibitor that is being developed by FibroGen, in collaboration with Astellas and AstraZeneca, for the treatment of anaemia in patients with dialysis-dependent chronic kidney disease (CKD), non-dialysis-dependent CKD and in patients with myelodysplastic syndromes.
Uses
Roxadustat is a hypoxia-inducible factor (HIF) prolyl hydroxylase inhibitor used to increase white blood cell levels in blood and hematopoietic progenitor cells in bone marrow.
Definition
ChEBI: Roxadustat is an N-acylglycine resulting from the formal condensation of the amino group of glycine with the carboxy group of 4-hydroxy-1-methyl-7-phenoxyisoquinoline-3-carboxylic acid. It is an inhibitor of hypoxia inducible factor prolyl hydroxylase (HIF-PH). It has a role as an EC 1.14.11.2 (procollagen-proline dioxygenase) inhibitor and an EC 1.14.11.29 (hypoxia-inducible factor-proline dioxygenase) inhibitor. It is a member of isoquinolines, an aromatic ether and a N-acylglycine.
Side effects
The most common side effects include hypertension, vascular access thrombosis (formation of blood clots in the blood vessels associated with dialysis), diarrhea, peripheral edema (swelling especially of the ankles and feet), hyperkalemia and nausea.
Synthesis
Preparation of roxadustat of formula 1 was described in a patent application WO2004108681, where the roxadustat is prepared in 11 steps from initial chemical compound 4-nitro-1,2-dicarbonitrile of formula 2. Reaction of dicarbonitrile of formula 2 with phenol and potash in the first step leads to preparation of corresponding phenoxy derivate of formula 3, 4-phenoxyphthalic acid of formula 4 is prepared by the following hydrolysis effect of potassium hydroxide in methanol, from which a solid-phase mixture with glycine is created for reaction in a melt with temperature of 210°C to 220°C, which provides corresponding phthalimide of formula 5. After that, phthalimide of formula 5 is esterified to corresponding methyl ester of formula 6. Then, expansion of the ring is performed by the effect of sodium butanolate to butyl-7-phenoxy-1,4-dihydroxy isoquinoline-3-carboxylate of formula 7. Hydroxyl group of derivative of formula 7 in a position 1 is then replaced by bromine using phosphorus oxybromide under the alkaline conditions resulting in creation of isoquinoline derivative of formula 8. Further the alkaline hydrolysis is carried out to provide an acid of formula 9. Then the lithiation is carried out, capture of lithium salt with methyl iodide, and in the second step, the hydroxyl group and carboxyl function are protected by benzyl group resulting in the derivative of formula 10. By the effect of potassium hydroxide the derivative of formula 10 is hydrolyzed to the carboxylic acid of formula 11, from which the derivative 12 is prepared under the conditions of glycine benzyl ester. The final step of synthesis of roxadustat of formula 1 is deprotection of benzyl groups by means of hydrogenation reaction (Diagram 1). 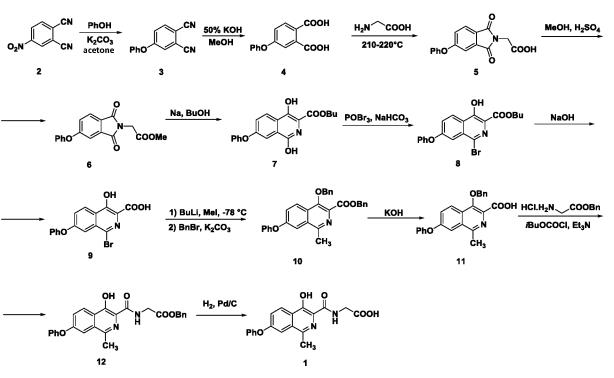
A Scalable Synthesis of Roxadustat (FG-4592)
IC 50
IC50 = 591.4 nM (HIF-PH).IC50 values for inhibition of peak and late components of delayed rectifier calcium currents are 5.71 and 1.32 μM in pituitary tumor cells.
Metabolism
Roxadustat is metabolized by cytochrome P450 (CYP)2C8 and uridine diphosphate-glucuronosyltransferase (UGT)1A9. Roxadustat is primarily metabolized through 2 main metabolic pathways: hydroxylation/oxidation followed by sulfation, producing metabolites 4'-hydroxy roxadustat and 4'-O-sulfate conjugates of 4'-hydroxy roxadustat, together accounting for 20% of the radioactive dose. The O-glucuronidation produces metabolite 4-O-β-glucuronide of roxadustat, representing 28% of the dose. Minor metabolic routes included glucosidation producing 4-O-β-glucoside of roxadustat (8.1% of the dose), acyl glucuronidation producing acyl-1-O-β-glucuronide of roxadustat (0.6% of the dose), and demethylation producing N-descarboxymethyl roxadustat oxide and Ndescarboxymethyl roxadustat (together ~3.6% of the dose).
Mode of action
Roxadustat is a novel, orally bioavailable, potent and reversible HIF-PH inhibitor (HIF-PHI) that transiently induces HIF stabilization and leads to a functional HIF transcriptional response that mimics the erythropoietic response associated with exposure of humans to intermittent hypoxia. roxadustat increases hemoglobin levels with a mechanism of action that is different from that of ESAs. Roxadustat activates the body’s natural protective response to reduced oxygen levels in the blood. This response involves the regulation of multiple, complementary processes that promote a coordinated erythropoietic response and increase the blood’s oxygen-carrying capacity.
Roxadustat Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Dr. Reddy's Laboratories Ltd | +91-4049002900 +91-4049002900 | Hyderabad, India | 165 | 58 | Inquiry |
| Lewens Labs | +917043057100 | Gujarat, India | 30 | 58 | Inquiry |
| Ami Lifesciences Pvt Ltd | +91-9426998100 +91-9426998100 | Gujarat, India | 62 | 58 | Inquiry |
| Maithri Drugs Pvt Ltd | +91-9059204566 +91-9848881740 | Telangana, India | 64 | 58 | Inquiry |
| MAITHRI DRUGS PRIVATE LTD | +91 40 30438600 | New Delhi, India | 29 | 58 | Inquiry |
| DR REDDYS LABORATORIES LTD | +91-40-49002900 | New Delhi, India | 201 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6257 | 58 | Inquiry |
| SynZeal Research Pvt Ltd | +1 226-802-2078 | Gujarat, India | 6514 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6739 | 58 | Inquiry |
| Shanghai Famo Biotech Co Ltd | +86-36680037 +86-18550473860 | China | 528 | 58 | Inquiry |
Related articles
- Roxadustat: Indications, Mechanism of Action and Side Effects
- Roxadustat is an oral, bioavailable and reversible inhibitor of hypoxia-inducible factor-prolinyl hydroxylases (HIF-PHIs). It ....
- Sep 5,2024
- An orally active HIF-PHD inhibitor: Roxadustat
- Roxadustat is an orally administered small molecule drug that prevents the enzymatic degradation of HIF-1α by inhibiting the P....
- Dec 25,2023
808118-40-3(Roxadustat)Related Search:
1of4
chevron_right




