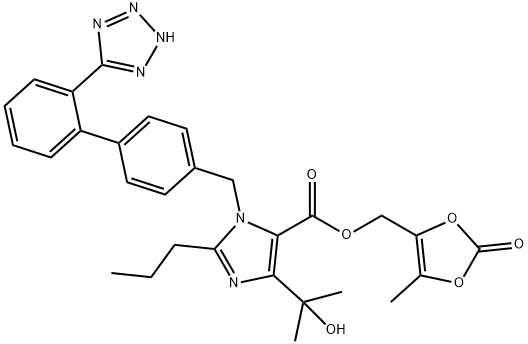
Olmesartan Medoxomil
- CAS No.
- 144689-63-4
- Chemical Name:
- Olmesartan Medoxomil
- Synonyms
- Benicar;Oimesartan;OLMESARTAN MEDOXIMIL;OMST;CS-866;Olmetec;Olmesartan API;Omesartan ester;Olmesartan Medoxomi;OlmesartanC29H30N6O6
- CBNumber:
- CB8254825
- Molecular Formula:
- C29H30N6O6
- Molecular Weight:
- 558.59
- MOL File:
- 144689-63-4.mol
- Modify Date:
- 2024/5/6 14:49:57
| Melting point | 180°C |
|---|---|
| Boiling point | 804.2±75.0 °C(Predicted) |
| Density | 1.38±0.1 g/cm3(Predicted) |
| Flash point | 180°C |
| storage temp. | 2-8°C |
| solubility | DMSO: soluble20mg/mL, clear |
| pka | 4.15±0.10(Predicted) |
| form | powder |
| color | white to beige |
| Decomposition | 180 ºC |
| InChIKey | UQGKUQLKSCSZGY-UHFFFAOYSA-N |
| SMILES | C1(CCC)N(CC2=CC=C(C3=CC=CC=C3C3=NNN=N3)C=C2)C(C(OCC2=C(C)OC(=O)O2)=O)=C(C(O)(C)C)N=1 |
| CAS DataBase Reference | 144689-63-4(CAS DataBase Reference) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302+H312+H332 | |||||||||
| Precautionary statements | P261-P264-P280-P301+P312-P302+P352+P312-P304+P340+P312 | |||||||||
| RTECS | NI4014200 | |||||||||
| HS Code | 2934990002 | |||||||||
| NFPA 704 |
|
Olmesartan Medoxomil price More Price(5)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | SML1391 | Olmesartan medoxomil ≥98% (HPLC) | 144689-63-4 | 50MG | ₹11539.45 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | SML1391 | Olmesartan medoxomil ≥98% (HPLC) | 144689-63-4 | 250MG | ₹45410.88 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | PHR1851 | Olmesartan Medoxomil Pharmaceutical Secondary Standard; Certified Reference Material | 144689-63-4 | 200MG | ₹22743.33 | 2022-06-14 | Buy |
| TCI Chemicals (India) | O0510 | Olmesartan Medoxomil | 144689-63-4 | 1G | ₹6100 | 2022-05-26 | Buy |
| TCI Chemicals (India) | O0510 | Olmesartan Medoxomil | 144689-63-4 | 5G | ₹9800 | 2022-05-26 | Buy |
Olmesartan Medoxomil Chemical Properties,Uses,Production
Description
Olmesartan medoxomil was launched in the US as benicar(R), an orally administered treatment for hypertension. Olmesartan, is a new selective and competitive nonpeptide angiotensin II type 1 receptor antagonist and potently inhibits the Ang.ll-induced pressor responses. The drug competitively inhibited binding of [125I1]-All to AT1 receptors in bovine adrenal cortical membranes, but had no effect on binding to AT2 receptors in bovine cerebellar membranes. In comparative clinical studies in patients with essential hypertension, olmesartan reduced sitting cuff diastolic blood pressure significantly more than losartan, valdesartan and ibesartan, while reductions in systolic blood pressure were similar for all treatments. Olmesartan medoxomil was also shown to reduce blood pressure significantly more effectively than losartan and the ACE inhibitor captopril and as effectively as the pbloker atenolol.
Chemical Properties
White to off-white crystalline powder
Uses
Olmesartan medoxomil is an angiotensin II receptor antagonist used to treat high blood pressure. Olmesartan works by blocking the binding of angiotensin II to the AT1 receptors in vascular muscle. By blocking the binding rather than the synthesis of angiotensin II, olmesartan inhibits the negative regulatory feedback on renin secretion.
Olmesartan medoxomil is a pro-drug that is de-esterified to the active metabolite, olmesartan. Olmesartan has a dual method of elimination, with about 60% eliminated by the liver and the remainder by the kidney. In situations of impaired renal or hepatic function, the alternative excretion pathway can compensate for the compromised one. Olmesartan is not metabolized by the cytochrome P450 enzyme system and therefore has a low potential for metabolic drug interactions, a feature that may be of importance when treating patients on multiple drug regimens, such as the elderly. Olmesartan is well tolerated and has an excellent safety profile that is comparable to that of placebo. In addition, olmesartan provides 24-h blood pressure control with a once-daily dosing. In head-to-head studies, olmesartan delivered superior blood pressure reduction when compared with other angiotensin-II receptor antagonists at their recommended doses.
General Description
Olmesartan Medoxomil is a synthetic imidazole derivative prodrug with an antihypertensive property. Upon hydrolysis, olmesartan medoxomil is converted to olmesartan. Olmesartan selectively binds to the angiotensin type 1 (AT1) receptor of angiotensin II in vascular smooth muscle and adrenal gland, thereby competing angiotensin II binding to the receptor. This prevents angiotensin II-induced vasoconstriction and decreases aldosterone production, thereby preventing aldosterone-stimulated sodium retention and potassium excretion.
Side effects
Dizziness or lightheadedness may occur as your body adjusts to the medication. If any of these effects persist or worsen, tell your doctor or pharmacist promptly. To reduce the risk of dizziness and lightheadedness, get up slowly when rising from a sitting or lying position.
Synthesis
Olmesartan Medoxomil can be synthesized in 8 steps from diaminomaleonitrile by successive reactions with trialkylorthopropanoate to access 2-propyl-imidazole-45dicarbonitrile, conversion of the two nitrile functions to the corresponding ethyl esters, followed by methylmagnesium bromide addition to give the corresponding 4-(1-hydroxyalkyl)imidazole derivative. 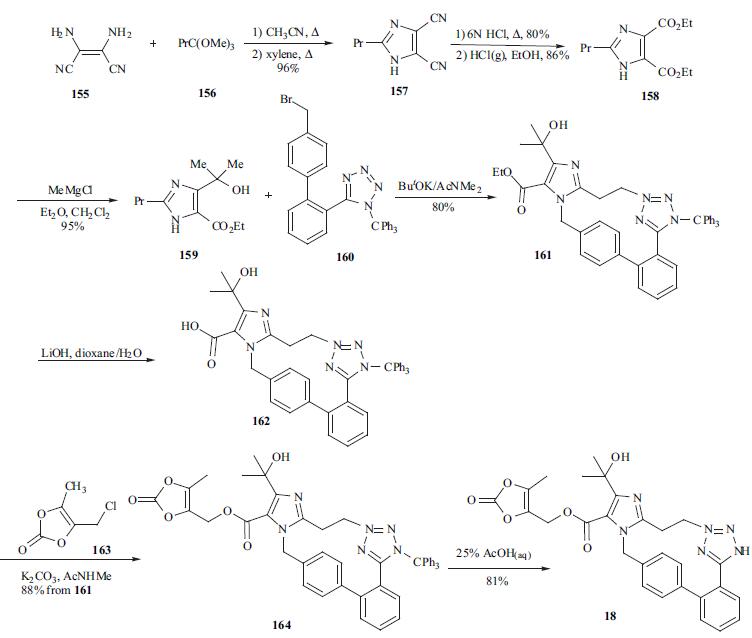
The imidazole ring of olmesartan (18) was constructed with diaminomaleonitrile 155 and trimethylorthobutyrate (156) in CH3CN then xylene to give 157 in 96% yield. Acid hydrolysis of 157 in 6N HCl gave the dicarboxylic acid intermediate. After esterification of the diacid in ethanol in the presence of HCl, diester 158 was treated with MeMgCl to give 4-(1-hydroxyalkyl) imidazole 159 in 95% yield. Alkylation of 159 with biphenyl bromide 160 in the presence of potassium tbutoxide afforded 161 in 80% yield. Ester 161 was then hydrolyzed to free carboxylic acid 162 under basic conditions, and 162 was treated with chloride 163 in the presence of K2CO3 to give ester 164 in 88% yield from 161.Lastly, the trityl group was removed with 25% aqueous acetic acid to give olmesartan (18) in 81% yield.
Olmesartan Medoxomil Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| GLR Innovations | +91 9891111994 | New Delhi, India | 4542 | 58 | Inquiry |
| Cadila Pharmaceuticals Ltd | +91-7069076657 +91-7069076657 | Ahmedabad, India | 54 | 58 | Inquiry |
| SGMR PHARMACEUTICALS PVT LTD | +91-9032001889 +91-9032001889 | Telangana, India | 83 | 58 | Inquiry |
| ANWITA APIS | +919000311012 | Hyderabad, India | 198 | 58 | Inquiry |
| ChemBioReas Life Sciences | +91-7989264158 +91-7989264158 | Hyderabad, India | 82 | 58 | Inquiry |
| SEUTIC | +91-8309787199 +91-8309787199 | Hyderabad, India | 124 | 58 | Inquiry |
| Prajna generics pvt ltd | +91-9441174873 +91-9441174873 | Hyderabad, India | 24 | 58 | Inquiry |
| Lewens Labs | +917043057100 | Gujarat, India | 30 | 58 | Inquiry |
| Cefa-Cilinas Biotics Pvt Ltd | +91-7875033155 +91-8080701561 | Maharashtra, India | 121 | 58 | Inquiry |
| KARPSCHEM LABORATORIES | +91-7249203006 +91-7249203006 | Maharashtra, India | 786 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| GLR Innovations | 58 |
| Cadila Pharmaceuticals Ltd | 58 |
| SGMR PHARMACEUTICALS PVT LTD | 58 |
| ANWITA APIS | 58 |
| ChemBioReas Life Sciences | 58 |
| SEUTIC | 58 |
| Prajna generics pvt ltd | 58 |
| Lewens Labs | 58 |
| Cefa-Cilinas Biotics Pvt Ltd | 58 |
| KARPSCHEM LABORATORIES | 58 |
Related articles
- Olmesartan Medoxomil: Pharmacokinetics and Clinical Applications
- Olmesartan medoxomil, rapidly converted to olmesartan, is a common angiotensin II receptor blocker effective in treating hyper....
- Mar 6,2024
- Efficacy and safety of Olmesartan Medoxomil in the treatment of hypertension
- Olmesartan medoxomil is an orally administered angiotensin II receptor antagonist, selective for the angiotensin II type 1 rec....
- Jan 5,2024
- Olmesartan medoxomil: pharmacological properties, therapeutic efficacy and tolerability
- Olmesartan medoxomil is an effective and safe ARB that lowers blood pressure, improves arterial compliance, and protects again....
- Sep 27,2023
144689-63-4(Olmesartan Medoxomil)Related Search:
1of4
chevron_right




