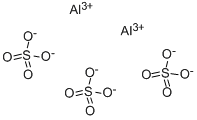
Aluminum sulfate
- CAS No.
- 10043-01-3
- Chemical Name:
- Aluminum sulfate
- Synonyms
- ALUMINIUM SULPHATE;ALUMINIUM SULFATE;ALUM;Aluminum(III) sulfate;ALUMINIUM SULPAHTE;ALUMINIUM SULPHATE 17% MIN;NA-9078;pearlalum;liusuanlv;filteralum
- CBNumber:
- CB8435192
- Molecular Formula:
- Al2O12S3
- Molecular Weight:
- 342.15
- MOL File:
- 10043-01-3.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/5/21 21:51:03
| Melting point | 770 °C (dec.) (lit.) |
|---|---|
| Boiling point | 759.71°C (estimate) |
| Density | 2.71 g/mL at 25 °C (lit.) |
| vapor pressure | 0-0.001Pa at 20-25℃ |
| solubility | Soluble in cold water, freely soluble in hot water, practically insoluble in ethanol (96 per cent). |
| pka | 3.6[at 20 ℃] |
| form | Powder and/or Chunks |
| Specific Gravity | 2.71 |
| color | White |
| Odor | at 100.00?%. odorless |
| Water Solubility | soluble |
| Sensitive | Hygroscopic |
| Merck | 14,366 |
| Exposure limits | NIOSH: TWA 2 mg/m3 |
| Dielectric constant | 2.6(Ambient) |
| LogP | -5.08--0.12 at 20℃ |
| CAS DataBase Reference | 10043-01-3(CAS DataBase Reference) |
| EPA Substance Registry System | Aluminum sulfate (10043-01-3) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS05 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H290-H318 | |||||||||
| Precautionary statements | P234-P280-P305+P351+P338-P390 | |||||||||
| Hazard Codes | Xi,N | |||||||||
| Risk Statements | 37/38-41-51/53-36/37/38 | |||||||||
| Safety Statements | 26-39-61-37/39-29 | |||||||||
| RIDADR | UN 1760/3077 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | BD1700000 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 28332200 | |||||||||
| Toxicity | guinea pig,LD50,unreported,490mg/kg (490mg/kg),Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 43(4), Pg. 12, 1978. | |||||||||
| NFPA 704 |
|
Aluminum sulfate price More Price(10)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 202614 | Aluminum sulfate 99.99% trace metals basis | 10043-01-3 | 5G | ₹6083.65 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 202614 | Aluminum sulfate 99.99% trace metals basis | 10043-01-3 | 5G | ₹6083.65 | 2022-06-14 | Buy |
| Sigma-Aldrich | 202614 | Aluminum sulfate 99.99% trace metals basis | 10043-01-3 | 25G | ₹20210.28 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 202614 | Aluminum sulfate 99.99% trace metals basis | 10043-01-3 | 25G | ₹20210.28 | 2022-06-14 | Buy |
| Sigma-Aldrich | 202614 | Aluminum sulfate 99.99% trace metals basis | 10043-01-3 | 100G | ₹37519.45 | 2022-06-14 | Buy |
Aluminum sulfate Chemical Properties,Uses,Production
Chemical Properties
Aluminum sulfate is a white powder, often used in water solution. The solution is a strong acid
Physical properties
White powder; refractive index 1.47; density 2.71 g/cm3; mp 770°C (decomposes); hygroscopic; readily soluble in water (31% at 0°C; solubility increases with temperature 98% in boiling water); soluble in dilute mineral acids; slightly soluble in alcohol.
Occurrence
It occurs in nature in minerals; alunite, KAl3(SO4)2(OH)6and natroalunite, NaAl3(SO4)2(OH)6. The anhydrous salt is used in food applications.
Uses
Aluminum sulfate acts as a flocculating agent in the purification of drinking water, and in waste water treatment plants. It acts as a mordant in dyeing, printing, textiles and also used in paper manufacturing. It is a waterproofing agent and accelerator in concrete. Further, it is used as a foaming agent in fire fighting foam, photographic film and in photochemicals. It is also used in styptic pencils, pain relief and in dentistry for gingival retraction cords.
Preparation
The anhydrous salt may be obtained by slow and progressive heating of commercial hydrated salt, Al2(SO4)3 ?18H2O. Most water molecules are lost at heating between 250 to 420°C. The last three water molecules are lost between 250 to 420°C at a heating rate of 10°C/min.
Definition
ChEBI: An aluminium sulfate that contains no water of crystallisation.
General Description
Anhydrous aluminum sulfate is a white crystalline solid. Aluminum sulfate is also obtained as an 18-hydrate Al2(SO4)3.18H2O. Both forms are soluble in water, noncombustible, and nontoxic. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. Aluminium sulfate is used in paper making, in firefighting foams, and in sewage treatment and water purification.
Air & Water Reactions
Dissolves in water with evolution of some heat. creates acidic solutions.
Reactivity Profile
Aqueous solutions of ALUMINUM SULFATE are acidic. The solid may corrode metals in presence of moisture.
Health Hazard
Inhalation of dust irritates nose and mouth. Ingestion of large doses causes gastric irritation, nausea, vomiting, and purging. Dust irritates eyes and skin.
Agricultural Uses
Alunogenite is a naturally occurring form of hydrated aluminum sulphate Al2(SO4)318 H2O.
Industrial uses
Aluminum chloride (AlCl3) can be obtained by reacting carbon dioxide and chlorine with kaolin at high temperatures. It is highly hygroscopic with a specific gravity of 2.3. It is highly soluble in water and in organic solvents. Similar to aluminum sulfate, aluminum chloride is used as a co-depressant for calcite, fluorite and dolomite.
Safety Profile
Moderately toxic by ingestion and intraperitoneal routes. Experimental reproductive effects. Human mutation data reported. Hydrolyzes to form sulfuric acid, whch irritates tissue, especially lungs. When heated to decomposition it emits toxic fumes of SOx,.
Potential Exposure
Widely used in the paper industry, in waste and water treatment and treating sewage; in antiperspirants, deodorants; in flame-retardants; in tanning leather, sizing paper; mordant in dyeing, purifying water, waterproofing cloth, clarifying oils and fats; in agricultural pesticides; manufacturing aluminum salts and others
Shipping
UN3264 Corrosive liquid, acidic, inorganic, n.o.s., Hazard class: 8; Labels: 8-Corrosive material, Technical Name Required. UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required
Purification Methods
It crystallises from hot dilute H2SO4 (l mL/g) on cooling in ice. When a solution of alumina (Al2O3) in conc H2SO4 is slowly cooled, Al2SO4 17 or 18H2O deposits as a crystalline mass. Al2SO4 17H2O is the stable form in equilibrium with its saturated aqueous solution at 25o [Smith J Am Chem Soc 64 41 1942]. This is purified by dissolving it in a small volume of H2O and adding EtOH until the sulfate readily crystallises from the oily supersaturated solution. It forms Al2O3 16H2O between 0-112o. On gradual heating, the hydrate melts, giving the anhydrous salt at ca 250o. Several hydrates up to 27H2O have been described. Further heating to red heat (~ 600-800o) causes decomposition to Al2O3 + SO3 + SO2 and O2 [Cobb J Soc Chem Ind 29 250 1910]. The ACS reagent is Al2O3 18H2O (98+%).
Incompatibilities
In aqueous solution, aluminum sulfate forms sulfuric acid; reacts with bases and many other substances. Corrodes metals, some plastics and body tissues, especially in the presence of moisture.
Waste Disposal
Pretreatment involves hydrolysis followed by neutralization with NaOH. The insoluble aluminum hydroxide formed is removed by filtration and can be heated to decomposition to yield alumina which has valuable industrial applications. The neutral solution of sodium sulfate can be discharged into sewers and waterways as long as its concentration is below the recommended provisional limit of 250 mg/L
Aluminum sulfate Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
1of8
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| sheetalchemicals | +91-9820130159 +91-9820130159 | Maharashtra, India | 77 | 58 | Inquiry |
| S.V ENTERPRISES | +919322701159 | Mumbai, India | 150 | 58 | Inquiry |
| UNILOSA INTERNATINAL PRIVATE LIMITED | +91-9999069917 +91-9999069917 | New Delhi, India | 152 | 58 | Inquiry |
| JSK Chemicals | +919879767970 | Gujarat, India | 3756 | 58 | Inquiry |
| Ultra Chemical Works | +91-9820078105 +91-9820078105 | Mumbai, India | 124 | 58 | Inquiry |
| Kronox Lab Sciences Pvt Ltd | +91-9313231074 +91-9313231074 | Gujarat, India | 240 | 58 | Inquiry |
| Vats International | +91-9810131850 +91-9810984000 | New Delhi, India | 19 | 58 | Inquiry |
| Agilenobel | +91-8090295395 +91-8800293647 | Haryana, India | 50 | 58 | Inquiry |
| Sujata Chemicals | +91-9898025496 +91-9898025496 | Gujarat, India | 73 | 58 | Inquiry |
| Snow White Products | +91-9820070100 +91-9820070100 | Maharashtra, India | 35 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| sheetalchemicals | 58 |
| S.V ENTERPRISES | 58 |
| UNILOSA INTERNATINAL PRIVATE LIMITED | 58 |
| JSK Chemicals | 58 |
| Ultra Chemical Works | 58 |
| Kronox Lab Sciences Pvt Ltd | 58 |
| Vats International | 58 |
| Agilenobel | 58 |
| Sujata Chemicals | 58 |
| Snow White Products | 58 |
Related articles
- A Comprehensive Review of Aluminum sulfate
- Aluminum sulfate, a widely used inorganic compound,from water treatment to paper production, This paper reviews the properties....
- May 21,2024
- Property and Application of Aluminum Sulfate
- Anhydrous aluminum sulfate is a white crystalline solid. Aluminum sulfate is also obtained as an 18-hydrate Al2(SO4)3.18H2O. B....
- Jul 7,2022
10043-01-3(Aluminum sulfate)Related Search:
1of4
chevron_right




