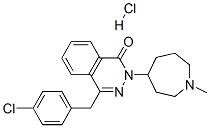
Azelastine hydrochloride
- CAS No.
- 79307-93-0
- Chemical Name:
- Azelastine hydrochloride
- Synonyms
- AZELASTINE HCL;Astelin;Optivar;1(2H)-Phthalazinone, 4-[(4-chlorophenyl)methyl]-2-(hexahydro-1-methyl-1H-azepin-4-yl)-, monohydrochloride;4-[(4-chlorophenyl)methyl]-2-(hexahydro-1-methyl-1H-azepin-4-yl)-1(2H)-phthalazinone, hydrochloride (1:1);Azen;a5610;e-0659;Azepit;w-2979m
- CBNumber:
- CB9256008
- Molecular Formula:
- C22H25Cl2N3O
- Molecular Weight:
- 418.36
- MOL File:
- 79307-93-0.mol
- MSDS File:
- SDS
- Modify Date:
- 2025/1/27 9:38:02
| Melting point | 225--2290C |
|---|---|
| storage temp. | Inert atmosphere,Store in freezer, under -20°C |
| solubility | DMSO: >10mg/mL |
| form | powder |
| color | white to off-white |
| Merck | 14,906 |
| Stability | Incompatible with strong oxidizing agents. |
| InChI | InChI=1S/C22H24ClN3O.ClH/c1-25-13-4-5-18(12-14-25)26-22(27)20-7-3-2-6-19(20)21(24-26)15-16-8-10-17(23)11-9-16;/h2-3,6-11,18H,4-5,12-15H2,1H3;1H |
| InChIKey | YEJAJYAHJQIWNU-UHFFFAOYSA-N |
| SMILES | C1(=O)C2=C(C=CC=C2)C(CC2=CC=C(Cl)C=C2)=NN1C1CCCN(C)CC1.[H]Cl |
| CAS DataBase Reference | 79307-93-0(CAS DataBase Reference) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302 | |||||||||
| Precautionary statements | P301+P312+P330 | |||||||||
| Hazard Codes | Xn | |||||||||
| Risk Statements | 22 | |||||||||
| RTECS | TH9203900 | |||||||||
| HS Code | 2933992600 | |||||||||
| Toxicity | LD50 in male, female mice, male, female rats (mg/kg): 36.5, 35.5, 26.9, 30.3 i.v.; 56.4, 42.8, 43.2, 46.6 i.p.; 63.0, 54.2, 66.5, 59.6 s.c.; 124, 139, 310, 417 orally (Zechel) | |||||||||
| NFPA 704 |
|
Azelastine hydrochloride price More Price(5)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | A7611 | Azelastine hydrochloride ≥98% (HPLC) | 79307-93-0 | 10MG | ₹8346.08 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | PHR1636 | Azelastine Hydrochloride Pharmaceutical Secondary Standard; Certified Reference Material | 79307-93-0 | 1G | ₹18348.38 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | A7611 | Azelastine hydrochloride ≥98% (HPLC) | 79307-93-0 | 50MG | ₹32929.65 | 2022-06-14 | Buy |
| TCI Chemicals (India) | A2340 | Azelastine Hydrochloride | 79307-93-0 | 100MG | ₹5000 | 2022-05-26 | Buy |
| TCI Chemicals (India) | A2340 | Azelastine Hydrochloride | 79307-93-0 | 1G | ₹10800 | 2022-05-26 | Buy |
Azelastine hydrochloride Chemical Properties,Uses,Production
Description
Azelastine hydrochloride is an orally effective antihistamine useful in the treatment of asthma and nasal allergy. It appears to inhibit release of histamine, in addition to antagonizing its action.
Chemical Properties
White or almost white, crystalline powder.
Uses
Orally active H1-hystamine receptor antagonist. Antihistaminic
Definition
ChEBI: The hydrochloride salt of azelastine.
Application
Azelastine Hydrochloride is the hydrochloride salt form of azelastine, a phthalazinone derivative with antihistaminergic activity. Azelastine hydrochloride exhibits histamine H1-receptor antagonist activity in isolated tissues, animal models, and humans. Azelastine hydrochloride nasal solution is administered as a racemic mixture, and no difference in pharmacologic activity was noted between the enantiomers in vitro studies. The major metabolite, desmethylazelastine, also possesses H1-receptor antagonist activity. It competes with histamine for the H1 receptor, thereby diminishing the actions of histamine on effector cells and decreasing the histamine-mediated symptoms of allergic reaction, such as bronchoconstriction, vasodilation, increased capillary permeability and spasmodic contractions of gastrointestinal smooth muscle.
General Description
(±)-4-[(4-chlorophenyl)methyl]-2-(hexahydro-l-methyl-1H-azepin-4-yl)-l-(2H)-phthalazinone monohydrochloride (Optivar), is a whitecrystalline powder that is sparingly soluble in water,methanol, and propylene glycol and slightly soluble inethanol, octanol, and glycerine. The commercial preparationis available as a 0.05% sterile ophthalmic solution fortopical administration to the eyes. Each milliliter of azelastinesolution contains 0.5-mg azelastine hydrochlorideequivalent to 0.457 mg of azelastine base, the preservativebenzalkonium chloride (0.125 mg), and inactive ingredientsincluding disodium edetate dihydrate, hydroxypropylmethylcellulose,sorbitol solution, sodium hydroxide, andwater for injection. The solution has a pH of approximately5.0 to 6.5 and an osmolality of approximately 271to 312 mOsm/L.
The recommended dose of azelastine solution is 1 dropinstilled into each affected eye twice a day. This drugproduct is for ocular administration only and not for injectionor oral use. Absorption of azelastine following ocularadministration is relatively low (less than 1 ng/rnL).Absorbed drug undergoes extensive oxidative N-demethylationby CYP, and the parent drug and metabolite are eliminatedprimarily in the feces. The most frequently reportedadverse reactions are transient eye burning or stinging,headaches, and bitter taste. Azelastine solution should beused with caution during pregnancy or while nursing, becauseits safety has not been studied under these circumstances.
Safety Profile
Poison by ingestion andintravenous routes. An experimental teratogen. Otherexperimental reproductive effects. When heated todecomposition it emits toxic fumes of NOx and HCl.
Azelastine hydrochloride Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Synthetic Research Pharma Laboratories Pvt Ltd | +91 9848023377 | Hyderabad, India | 116 | 58 | Inquiry |
| Coral Drugs Pvt Ltd | +91-1244518100 +91-8199986118 | New Delhi, India | 24 | 58 | Inquiry |
| Vamsi Labs Ltd | +91-2172357274 +91-9891966300 | Maharashtra, India | 43 | 58 | Inquiry |
| Zydus Lifesciences Ltd. | +91-0265-2315243 +91-6358895661 | Gujarat, India | 89 | 58 | Inquiry |
| Ralington Pharma | +91-7948911722 +91-9687771722 | Gujarat, India | 1350 | 58 | Inquiry |
| BDR Pharmaceuticals International Pvt Ltd | +91-2240560560 +91-7718884418 | Maharashtra, India | 206 | 58 | Inquiry |
| Turtle Pharma Private Limited | +91-9850998504 +91-9850998504 | Maharashtra, India | 27 | 58 | Inquiry |
| TIKVAH PHARMA SOLUTIONS PVT LTD | +918341143995 | Hyderabad, India | 46 | 58 | Inquiry |
| Mesha Pharma Private Limited | 08048372569Ext 806 | Mumbai, India | 133 | 58 | Inquiry |
| Ayon PharmaChem | 91-22-28638348 | Maharashtra, India | 303 | 58 | Inquiry |
Related articles
- What is an Azelastine hydrochloride nasal solution?
- Azelastine hydrochloride (Astelin) nasal spray 0.1% solution is a second-generation intranasal antihistamine available in the ....
- Jul 12,2024
79307-93-0(Azelastine hydrochloride)Related Search:
1of4
chevron_right




