Upadacitinib
|
- ₹0
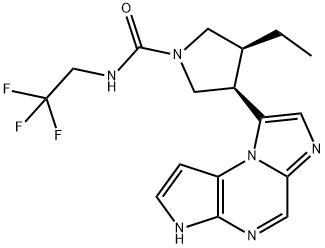
- Product name: Upadacitinib
- CAS: 1310726-60-3
- MF: C17H19F3N6O
- MW: 380.37
- EINECS:213-161-9
- MDL Number:MFCD30502663
- Synonyms:Upadacitinib;ABT-494(Upadacitinib) free base;ABT-494 (Upadacitinib);Upadacitinib (Rinvoq);ABT-494; ABT494; ABT 494;CS-2730;Upacidatinib;ABT-494
| Manufacturer | Product number | Product description | Packaging | Price | Updated | Buy |
|---|
Properties
Density :1.56±0.1 g/cm3(Predicted)
storage temp. :Store at -20°C
solubility :DMSO:42.67(Max Conc. mg/mL);112.17(Max Conc. mM)
DMSO:PBS (pH 7.2) (1:1):0.5(Max Conc. mg/mL);1.31(Max Conc. mM)
DMF:30.0(Max Conc. mg/mL);78.87(Max Conc. mM)
Ethanol:76.0(Max Conc. mg/mL);199.8(Max Conc. mM) form :A crystalline solid
pka :11.89±0.60(Predicted)
color :White to off-white
InChI :InChI=1S/C17H19F3N6O/c1-2-10-7-25(16(27)24-9-17(18,19)20)8-11(10)13-5-22-14-6-23-15-12(26(13)14)3-4-21-15/h3-6,10-11,21H,2,7-9H2,1H3,(H,24,27)/t10-,11+/m1/s1
InChIKey :WYQFJHHDOKWSHR-MNOVXSKESA-N
SMILES :N1(C(NCC(F)(F)F)=O)C[C@H](C2N3C4C=CNC=4N=CC3=NC=2)[C@H](CC)C1
storage temp. :Store at -20°C
solubility :DMSO:42.67(Max Conc. mg/mL);112.17(Max Conc. mM)
DMSO:PBS (pH 7.2) (1:1):0.5(Max Conc. mg/mL);1.31(Max Conc. mM)
DMF:30.0(Max Conc. mg/mL);78.87(Max Conc. mM)
Ethanol:76.0(Max Conc. mg/mL);199.8(Max Conc. mM) form :A crystalline solid
pka :11.89±0.60(Predicted)
color :White to off-white
InChI :InChI=1S/C17H19F3N6O/c1-2-10-7-25(16(27)24-9-17(18,19)20)8-11(10)13-5-22-14-6-23-15-12(26(13)14)3-4-21-15/h3-6,10-11,21H,2,7-9H2,1H3,(H,24,27)/t10-,11+/m1/s1
InChIKey :WYQFJHHDOKWSHR-MNOVXSKESA-N
SMILES :N1(C(NCC(F)(F)F)=O)C[C@H](C2N3C4C=CNC=4N=CC3=NC=2)[C@H](CC)C1
Safety Information
| Symbol(GHS): |

|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Signal word: | Warning | ||||||||||||||
| Hazard statements: |
|
||||||||||||||
| Precautionary statements: |
|
Description
Upadacitinib is a JAK1 inhibitor (IC50 = 47 nM). It is selective for JAK1 over JAK3 and tyrosine kinase 2 (Tyk2; IC50s = 2,304 and 4,690 nM, respectively), as well as a panel of 83 additional kinases at 1 μM, but does inhibit JAK2, Rho-associated kinase I (ROCK1), and ROCK2 (IC50s = 120, 920, and 430 nM, respectively). Upadacitinib decreases cytokine-induced STAT phosphorylation in a variety of human cells with IC50 values ranging from 1.6 to 649 nM. It reduces M. tuberculosis-induced paw swelling and bone erosion in a rat model of arthritis when administered at doses of 1, 3, and 10 mg/kg twice per day for 17 days. Formulations containing upadacitinib have been used in the treatment of rheumatoid arthritis.More related product prices
Ibrutinib Baricitinib tert-butyl 5-tosyl-5H-pyrrolo[2,3-b]pyrazin-2-ylcarbamate Senexin B 1-((2S,5R)-5-((7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)-2-methylpiperidin-1-yl)prop-2-en-1-one H3B-6527 N-(2-(4'-cyanobiphenyl-4-yl)propyl)propane-2-sulfonaMide Q-VD-OPh hydrate 321674-73-1 3-(1-Methyl-1H-pyrazol-4-yl)-5-oxo-N-(2-pyridinylmethyl)-5H-benzo[4,5]cyclohepta[1,2-b]pyridine-7-methanesulfonamide Vandetanib Olaparib Nintedanib Ethanesulfonate Salt AZD-9291Related product price
- 1-((2S,5R)-5-((7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)-2-methylpiperidin-1-yl)prop-2-en-1-one
₹10954.9-44057.75 - Q-VD-OPh hydrate
₹25644.43-103865.88 - 321674-73-1
₹17660.01
Suppliers and manufacturers
Venkatasai Life Sciences
A.J Chemicals
CLEARSYNTH LABS LTD.
Pharmaffiliates Analytics and Synthetics P. Ltd
SynZeal Research Pvt Ltd
Shanghai Rochi Pharmaceutical Co.,Ltd.
Beijing Mesochem Technology Co.,Ltd
TAIZHOU YUXIN BIOTECHNOLOGY CO,.LTD
Wuhan Topule Biopharmaceutical Co., Ltd
SHANDONG BOYUAN PHARMACEUTICAL CO., LTD.






