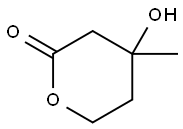
DL-Mevalonolactone
- CAS No.
- 674-26-0
- Chemical Name:
- DL-Mevalonolactone
- Synonyms
- NSC 90804;Beta-Hydro;Hyperpure-MVA;MEVALONOLACTONE;DL-MEVALOLACTONE;MEVALONIC LACTONE;DL-MEVALONOLACTONE;(+/-)-MEVALONOLACTONE;Adeka Mevalonolactone;DL-Mevalonolactone >
- CBNumber:
- CB0377087
- Molecular Formula:
- C6H10O3
- Molecular Weight:
- 130.14
- MOL File:
- 674-26-0.mol
- Modify Date:
- 2024/5/14 17:56:46
| Melting point | 28 °C(lit.) |
|---|---|
| Boiling point | 145-150 °C5 mm Hg(lit.) |
| alpha | -1.0~+1.0°(20℃/D)(c=5, C2H5OH) |
| Density | 1.1066 (rough estimate) |
| refractive index |
n |
| Flash point | >230 °F |
| storage temp. | -20°C |
| solubility | Chloroform (Sparingly), DMSO (Slightly), Ethyl Acetate, Methanol (Slightly) |
| form | Oil |
| pka | 13.57±0.20(Predicted) |
| color | Pale Yellow to Dark Brown |
| Merck | 14,6163 |
| BRN | 80960 |
| Stability | Hygroscopic |
| InChIKey | JYVXNLLUYHCIIH-UHFFFAOYSA-N |
| CAS DataBase Reference | 674-26-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Dl-mevalonic acid lactone(674-26-0) |
DL-Mevalonolactone price More Price(4)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | M4667 | (±)-Mevalonolactone ~97% (titration) | 674-26-0 | 1G | ₹13098.25 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | M4667 | (±)-Mevalonolactone ~97% (titration) | 674-26-0 | 5G | ₹37887.5 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | M4667 | (±)-Mevalonolactone ~97% (titration) | 674-26-0 | 10G | ₹51678.55 | 2022-06-14 | Buy |
| TCI Chemicals (India) | D0587 | DL-Mevalonolactone min. 98.0 % | 674-26-0 | 100MG | ₹3200 | 2022-05-26 | Buy |
DL-Mevalonolactone Chemical Properties,Uses,Production
Description
DL-Mevalonolactone is the δ-lactone form of mevalonic acid, a precursor in the mevalonate pathway. It induces depolarization and swelling, decreases NADPH content and aconitase (ACO) activity, and increases malondialdehyde (MDA) production in calcium-loaded rat brain mitochondria. DL-Mevalonolactone reverses decreases in glucose uptake induced by simvastatin (Item Nos. 10010344 | 10010345) in L6 myotubes. Tissue levels and urinary excretion of DL-mevalonolactone are increased in patients with mevalonic aciduria, a disorder characterized by a deficiency in mevalonic kinase activity.
Chemical Properties
Pale Yellow Oil
Uses
A metabolite from endophytes of the medicinal plant Erythrina crista-galli.
General Description
Alkaline hydrolysis of mevalonolactone gives mevalonate. Mevalonate is a precursor of farnesyl and geranylgeranyl pyrophosphates. These pyrophosphates are required for protein prenylation.
Purification Methods
Purify the lactone via the dibenzyl-ethylenediammonium salt (m 124-125o) [Hofmann et al. J Am Chem Soc 79 2316 1957], or by chromatography on paper or on a Dowex-1 (formate) column. [Bloch et al. J Biol Chem 234 2595 1959.] Store it as the N,N'-dibenzylethylenediamine (DBED) salt, or as the lactone in a sealed container at 0o. [Beilstein 18/1 V 19.]
DL-Mevalonolactone Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| TCI Chemicals (India) Pvt. Ltd. | 1800 425 7889 | New Delhi, India | 6778 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Clearsynth Labs | 91-22-45045900 | Maharashtra, India | 3889 | 58 | Inquiry |
| Alfa Aesar | 1 800 209 7001 | Maharashtra, India | 6913 | 58 | Inquiry |
| Hebei Yanxi Chemical Co., Ltd. | +86-17531190177; +8617531190177 | China | 5873 | 58 | Inquiry |
| Wuhan Quanjinci New Material Co.,Ltd. | +8615271838296 | China | 1534 | 58 | Inquiry |
| airuikechemical co., ltd. | +undefined86-15315557071 | China | 983 | 58 | Inquiry |
| BINBO BIOLOGICAL CO.,LTD | +8618629063126 | China | 346 | 58 | Inquiry |
| Accela ChemBio Inc. | +1-858-6993322 | United States | 19479 | 58 | Inquiry |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | China | 49392 | 58 | Inquiry |
674-26-0(DL-Mevalonolactone)Related Search:
1of4
chevron_right




