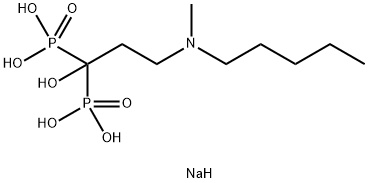
Ibandronate sodium
- CAS No.
- 138844-81-2
- Chemical Name:
- Ibandronate sodium
- Synonyms
- Bondronat;Ibandronate sodium;IBANDRONATE USP/EP/BP;Sodium Ibandronate >IbandronicAcidSodiumSal;Ibandronic Acid Sodium Salt;Ibandronate sodiuM Bondronat;Ibandronate Sodium Anhydrous;Ibandronate sodium USP/EP/BP;(1-Hydroxy-3-(methylpentylamino)propylidene)bisphosphonic acid monosodium salt
- CBNumber:
- CB31261504
- Molecular Formula:
- C9H24NNaO7P2
- Molecular Weight:
- 343.23
- MOL File:
- 138844-81-2.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/5/14 17:56:46
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302 |
| Precautionary statements | P264-P270-P301+P312-P330-P501 |
| WGK Germany | 3 |
| RTECS | SZ8563300 |
| HS Code | 29319090 |
Ibandronate sodium price More Price(4)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | I5784 | Ibandronate sodium salt ≥97% (NMR), solid | 138844-81-2 | 10MG | ₹12048.23 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | I5784 | Ibandronate sodium salt ≥97% (NMR), solid | 138844-81-2 | 50MG | ₹41080.88 | 2022-06-14 | Buy |
| TCI Chemicals (India) | S0877 | Sodium Ibandronate | 138844-81-2 | 1G | ₹8600 | 2022-05-26 | Buy |
| TCI Chemicals (India) | S0877 | Sodium Ibandronate | 138844-81-2 | 5G | ₹9800 | 2022-05-26 | Buy |
Ibandronate sodium Chemical Properties,Uses,Production
Description
ibandronate sodium (BONIVA) is a nitrogen-containing bisphosphonate that inhibits osteoclast-mediated bone resorption. The chemical name for ibandronate sodium is 3-(N-methyl-N-pentyl) amino-1-hydroxypropane-1,1-diphosphonic acid, monosodium salt, monohydrate with the molecular formula C9H22NO7P2Na?H20 and a molecular weight of 359.24. Ibandronate sodium is a white-to off-white powder. It is freely soluble in water and practically insoluble in organic solvents.
BONIVA is available as a white, oblong, 2.5-mg film-coated tablet for daily oral administration or as a white, oblong, 150-mg film-coated tablet for once-monthly oral administration. One 2.5-mg film-coated tablet contains 2.813 mg ibandronate monosodium monohydrate, equivalent to 2.5 mg free acid. One 150-mg film-coated tablet contains 168.75 mg ibandronate monosodium monohydrate, equivalent to 150 mg free acid. BONIVA also contains the following inactive ingredients: lactose monohydrate, povidone, microcrystalline cellulose, crospovidone, purified stearic acid, colloidal silicon dioxide, and purified water. The tablet film coating contains hypromellose, titanium dioxide, talc, polyethylene glycol 6000, and purified water.
Uses
Ibandronate sodium salt has been used to study its effect on the proliferation and ultrastructure of Leishmania and Giardia by the generation of concentration curves. It has also been used to elucidate the route by which nitrogen-containing bisphosphonates (N-BPs) enter the cytosol and inhibit their molecular target.
Pharmacokinetics
Its mechanism of action is identical to the other bisphosphonate agents. Administered daily (2.5 mg), ibandronate has been clinically shown to reduce the risk of vertebral fractures by 62%. If administered on an intermittent basis (20 mg), it reduces the risk of vertebral fractures by 50%. Ibandronate (2.5 mg daily), along with 500 mg of supplemental calcium, has been clinically shown to increase BMD in the hip (1.8%), femoral neck (2.0%), and lumbar spine (3.1%). The 150-mg formulation approved in March 2005 represents the first oral therapy for a chronic disease to be administered once monthly.
Clinical Use
Ibandronate sodium was approved in May 2003 for the treatment and prevention of osteoporosis in postmenopausal women.
Side effects
Adverse events as sociated with the injectable form ulation included arthralgia, back and abdominal pain, and hypertens ion. There is a risk of renal toxicity that is inversely related to the rate of administration of this formulation.
Metabolism
The oral bioavailability of this agent is extremely poor (0.6%) and is adversely affected by the presence of food, beverages other than water, and other medications, including calcium or vitamin D supplements and antacids. Because of the increased calcium content in mineral water, patients should not take this medication with this type of water. Drugs that inhibit gastric acid secretion (e.g., H2 antagonists and proton-pump inhibitors) actually promote ibandronate absorption. Like the others in this therapeutic class, ibandronate is not metabolized, and that which is not bound to the bone (40–50% of the absorbed dose) is eliminated renally unchanged. It does not inhibit the cytochrome P450 (CYP450) isozymes. This agent does not require any dosage adjustment for patients with hepatic impairment or mild to moderate renal impairment (creatinine clearance, >30 mL/min). Ibandronate should not be prescribed for patients with severe renal impairment (creatinine clearance, <30 mL/min).
Ibandronate sodium Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Dr. Reddy's Laboratories Ltd | +91-4049002900 +91-4049002900 | Hyderabad, India | 165 | 58 | Inquiry |
| Hetero Drugs Limited | +91-4023704923 +91-4023704923 | Telangana, India | 296 | 58 | Inquiry |
| Lee Pharma Ltd | +91-9849085929 +91-9849085929 | Hyderabad, India | 42 | 58 | Inquiry |
| Macleods Pharmaceuticals Limited | +91-2266762800 +91-2266762800 | Maharashtra, India | 116 | 58 | Inquiry |
| Orchid Pharma Ltd. (Formerly Known as Orchid Chemicals and Pharmaceuticals Ltd.) | +91-4428244254 +91-4428244254 | Tamil Nadu, India | 28 | 58 | Inquiry |
| NATCO Pharma Limited | +91-9849511345 +91-9866005199 | Hyderabad, India | 24 | 58 | Inquiry |
| Fleming Laboratories Ltd | +91-9666407333 +91-9666022445 | Hyderabad, India | 22 | 58 | Inquiry |
| Ralington Pharma | +91-7948911722 +91-9687771722 | Gujarat, India | 1350 | 58 | Inquiry |
| AKASH PHARMA EXPORTS | +91-9388123451 +91-9846039283 | Kerela, India | 470 | 58 | Inquiry |
| Athos Chemicals | 91-9998858909 | Gujarat, India | 315 | 58 | Inquiry |





