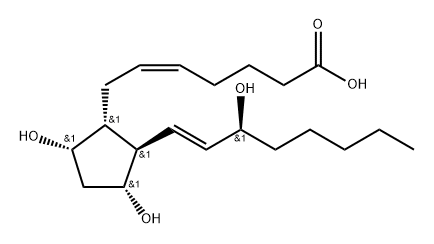
Prostaglandin F2a
- CAS No.
- 551-11-1
- Chemical Name:
- Prostaglandin F2a
- Synonyms
- glandin;u-14583;cyclosin;Dinprost;enzaprost;dinolytic;panacelan;Glandin N;DINOPROST;Dinaprost
- CBNumber:
- CB3161368
- Molecular Formula:
- C20H34O5
- Molecular Weight:
- 354.49
- MOL File:
- 551-11-1.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/7/2 8:55:05
| Melting point | 25-35° |
|---|---|
| alpha | D25 +23.5° (c = 1 in tetrahydrofuran) |
| Boiling point | 407.69°C (rough estimate) |
| Density | 1.0458 (rough estimate) |
| refractive index | 1.6120 (estimate) |
| storage temp. | Store at -20°C |
| solubility |
DMSO:100.0(Max Conc. mg/mL);282.1(Max Conc. mM) Water:100.0(Max Conc. mg/mL);282.1(Max Conc. mM) |
| pka | pKa 4.90(H2O,t=25±2,I=0.0,c<0.01,N2) (Uncertain) |
| form | Low melting white solid or colorless oil. |
| color | White to light brown |
| InChIKey | PXGPLTODNUVGFL-JPYXJAOSNA-N |
| SMILES | C([C@H]1[C@H](C[C@@H](O)[C@@H]1/C=C/[C@@H](O)CCCCC)O)/C=C\CCCC(=O)O |&1:1,2,4,6,9,r| |
| CAS DataBase Reference | 551-11-1(CAS DataBase Reference) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H302-H360D | |||||||||
| Precautionary statements | P201-P202-P264-P270-P301+P312-P308+P313 | |||||||||
| Toxicity | LD50 in rabbits (mg/kg): 2.5-5.0 i.v.; 2.5-5.0 i.m. (Fujita) | |||||||||
| NFPA 704 |
|
Prostaglandin F2a price More Price(3)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 538907 | Prostaglandin F 2α - CAS 551-11-1 - Calbiochem Major uterine luteolytic prostaglandin. | 551-11-1 | 1MG | ₹14100 | 2022-06-14 | Buy |
| TCI Chemicals (India) | P1885 | Prostaglandin F2α | 551-11-1 | 1MG | ₹3900 | 2022-05-26 | Buy |
| TCI Chemicals (India) | P1885 | Prostaglandin F2α | 551-11-1 | 10MG | ₹15000 | 2022-05-26 | Buy |
Prostaglandin F2a Chemical Properties,Uses,Production
Description
Prostaglandin F2α (PGF2α) is present in numerous species and has a widespread distribution. It can cause smooth muscle contraction in the vascular, bronchial, intestinal, and myometrial regions, and has potent luteolytic activity. PGF2α exerts its physiological effects through receptor mediation, with maximal ovine myometrial contraction being achieved at 125 nM PGF2α in vitro, and its receptor-mediated activity is most potent at 50-100 nM. Studies have also shown that PGF2α inhibits nitric oxide production in uterine tissue, enhances uterine contractility, and exhibits potent luteolytic activity. Furthermore, PGF2α functions as an activator of PGF2αR.
History
Prostaglandins (PGs) are a class of important endogenous products with a wide range of physiological activities. PGs were first discovered and named by American scholar Von Eluer in 1930. In 1962, Bergstorm extracted two pure PGs (PGF1 and PGF2) and determined their chemical structures. In 1969, Willis first proposed that PGs are an inflammatory mediator in the body. Subsequently, various physiological and pharmacological activities of PGs have been intensively studied.
Uses
Prostaglandin F2α is one of the most biologically studied of the primary prostaglandins. Closely related to Prostaglandin E2 (PGE2) in that both prostaglandins are biosynthesized from the same precursors and that PGF2 is the synthetic reduction product of PGE2.
Definition
ChEBI: Prostaglandin F2α is a prostaglandins Falpha that is prosta-5,13-dien-1-oic acid substituted by hydroxy groups at positions 9, 11 and 15. It is a naturally occurring prostaglandin used to induce labor.
Pharmacokinetics
Dinoprost is a natural prostaglandin F2α (PGF2α), which can directly act on the myometrium, stimulate the pregnant uterus to contract the uterine muscle, and can soften and dilate the cervix, so it can be used for induced abortion and late labor induction. However, due to the instability of dinoprost at room temperature, inconvenient storage and transportation, complex synthesis process and high cost, the application of dinoprost is difficult to popularize.
Safety Profile
Poison by subcutaneous, intravenous, and intramuscular routes. Moderately toxic by ingestion. Human and experimental teratogenic and experimental reproductive effects. Human reproductive effects by subcutaneous, intravenous, intramuscular, intraperitoneal, intravaginal, and intraplacental routes: postpartum depression and other maternal effects, abortion, and changes in measures of ferulity. Human teratogenic effects by intraplacental route: extra embryonic structures. Human systemic effects by intravenous route: hypermoulity, diarrhea, nausea or vomiting. Human mutation data reported. When heated to decomposition it emits acrid smoke and fumes
Mode of action
Prostaglandin F2α (PGF2α) stimulates myometrial activity, relaxes the cervix, inhibits corpus luteal steroidogenesis, and induces luteolysis by direct action on the corpus luteum. In pregnancy, PGF2α is medically used to sustain contracture and provoke myometrial ischemia to accelerate labor and prevent significant blood loss in labor.
Prostaglandin F2a Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| ANVI Pharma | +91-2228995761 +91-2228995761 | Maharashtra, India | 10 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| ANVI Pharma | 91-22-28995761 | Maharashtra, India | 4 | 58 | Inquiry |
| Clearsynth Labs | 91-22-45045900 | Maharashtra, India | 3889 | 58 | Inquiry |
| TCI Chemicals (India) Pvt. Ltd. | 1800 425 7889 | New Delhi, India | 6778 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| Sinoway Industrial co., ltd. | 0592-5800732; +8613806035118 | China | 990 | 58 | Inquiry |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | China | 21670 | 55 | Inquiry |
| BOC Sciences | +1-631-485-4226 | United States | 19553 | 58 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29826 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| ANVI Pharma | 58 |
| CLEARSYNTH LABS LTD. | 58 |
| ANVI Pharma | 58 |
| Clearsynth Labs | 58 |
| TCI Chemicals (India) Pvt. Ltd. | 58 |
| Pharmaffiliates Analytics and Synthetics P. Ltd | 58 |
| Sinoway Industrial co., ltd. | 58 |
| Henan Tianfu Chemical Co.,Ltd. | 55 |
| BOC Sciences | 58 |
| career henan chemical co | 58 |
551-11-1(Prostaglandin F2a)Related Search:
1of4
chevron_right




