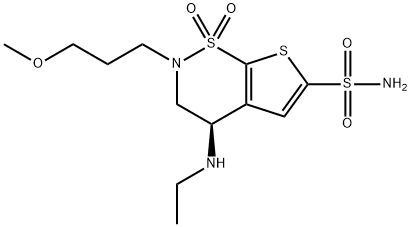
Brinzolamide
- CAS No.
- 138890-62-7
- Chemical Name:
- Brinzolamide
- Synonyms
- Azopt;AL 4;CT4.7;GAGE7;GAGE-7;GAGE-8;CS-138;GAGE-7B;AL 4862;AL-4682
- CBNumber:
- CB3458912
- Molecular Formula:
- C12H21N3O5S3
- Molecular Weight:
- 383.51
- MOL File:
- 138890-62-7.mol
- Modify Date:
- 2024/7/19 18:29:52
| Melting point | 130.0 to 134.0 °C |
|---|---|
| Boiling point | 586.0±60.0 °C(Predicted) |
| Density | 1.50±0.1 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | DMSO: ≥10mg/mL |
| pka | 9.62±0.40(Predicted) |
| form | powder |
| color | white to beige |
| Merck | 14,1376 |
| InChIKey | HCRKCZRJWPKOAR-JTQLQIEISA-N |
| CAS DataBase Reference | 138890-62-7(CAS DataBase Reference) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302 | |||||||||
| Precautionary statements | P264-P270-P301+P312+P330-P501 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | XJ9095055 | |||||||||
| HS Code | 2935904000 | |||||||||
| NFPA 704 |
|
Brinzolamide price More Price(4)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | SML0216 | Brinzolamide ≥98% (HPLC) | 138890-62-7 | 10MG | ₹12979.18 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | SML0216 | Brinzolamide ≥98% (HPLC) | 138890-62-7 | 50MG | ₹52133.2 | 2022-06-14 | Buy |
| TCI Chemicals (India) | B4258 | Brinzolamide | 138890-62-7 | 25MG | ₹4900 | 2022-05-26 | Buy |
| TCI Chemicals (India) | B4258 | Brinzolamide | 138890-62-7 | 100MG | ₹9200 | 2022-05-26 | Buy |
Brinzolamide Chemical Properties,Uses,Production
Description
Brinzolamide is a carbonic anhydrase inhibitor developed by Alcon (now Novartis) as a treatment for primary and open-angle glaucoma and ocular hypertension. It was First approval in the United States in 1998 under the trade name Azopt. It is the second of this class after dorzolamide (1995). Brinzolamide was approved as a generic medication in the United States in November 2020.Brinzolamide is a white powder commercially formulated as a 1% ophthalmic suspension to reduce intraocular pressure (IOP). In patients with primary open-angle glaucoma or ocular hypertension, brinzolamide produced significant reductions in IOP and showed less ocular discomfort than dorzolamide.
Chemical Properties
Brinzolamide has a molecular weight of 383.5 and a melting point of about 131°C. It is a white powder or crystalline Solid, which is insoluble in water, very soluble in methanol and soluble in ethanol.
Uses
Brinzolamide is a sulfonamide and carbonic anhydrase inhibitor with specific affinity for carbonic anhydrase II. Following topical ocular administration, brinzolamide inhibits carbonic anhydrase II, an enzyme that is responsible for the movement of sodium and fluid transport in the eye. This inhibition leads to a decrease in aqueous humor secretion, probably by slowing the formation of bicarbonate ions, and results in a reduction in intraocular pressure. Brinzolamide is used to treat increased pressure in the eye caused by open-angle glaucoma.
Uses
Brinzolamide has been used as a melanin binding compound or drug in melanin binding assays. It has also been used as a carbonic anhydrase inhibitor (CAI).
Indications
Brinzolamide, a heterocyclic sulfonamide, is a topical CAI suspension that has a high affinity for the carbonic anhydrase II isoenzyme.Because the ocular hypotensive effect of the drug is equivalent whether dosed twice or three times daily, brinzolamide 1% may be administered twice daily.
Definition
ChEBI: Brinzolamide is a sulfonamide and a thienothiazine. It has a role as an antiglaucoma drug and an EC 4.2.1.1 (carbonic anhydrase) inhibitor.
Preparation
Brinzolamide synthesis method: using thiophene as raw material, 3-acetyl-2,5-dichlorothiophene (4) is obtained by chlorination and acetylation, and 4 is reacted with sodium benzyl sulfide to obtain 6,6, which is chlorinated and ammoniated. Chemical and oxidation reactions "one-pot" synthesis of 7, Carbon-based α-hydrobromination of 7 with Pyridinium tribromide gives 9,9 is asymmetrically reduced under the action of (+)-Ipc2BCl to obtain 11, which is then subjected to N-alkylation and sulfonamidation to generate (S)-3,4-dihydro-4-hydroxy-2-(3-methoxyl propyl)-2H-thieno[3,2-e]-1,2-thiazine-6-sulfonamide-1,1-dioxide, the sulfonamide group was protected with trimethyl orthoacetate to give 15 , first introduce p-toluenesulfonyl group and then replace it with ethylamino group, and remove the sulfonamide group protecting group to obtain brinzolamide. The synthesis of intermediate 4 in this route is convenient, and each step of the reaction does not require column chromatography, and the total yield is 13.4%.
Graphical Synthetic Routes of Olanzapine
General Description
Brinzolamide is a small molecular weight compound that has an ability to bind melanin. This drug is used in ocular therapy.
Clinical Use
Brinzolamide is indicated for the treatment of elevated IOP in patients with ocular hypertension or open-angle glaucoma.The drug is commercially available as a sterile 1.0% aqueous suspension with a pH of approximately 7.5. BAC 0.01% is added as a preservative.
The efficacy and safety of brinzolamide 1%, either two or three times daily, were evaluated in 572 patients with open-angle glaucoma or ocular hypertension against timolol 0.5% twice daily and dorzolamide 2.0% three times daily. Mean IOP changes were -3.8 to -5.7 mm Hg, -4.2 to -5.6 mm Hg, and-4.3 to-5.9 mm Hg for two- and three-times-daily brinzolamide and dorzolamide dosing, respectively. The mean IOP changes for timolol 0.5% ranged from -5.6 to -6.3 mm Hg (Figure 10-15). Brinzolamide was well tolerated, with 1.8% (twice daily) and 3% (three times daily) of patients reporting ocular discomfort versus 16.4% with dorzolamide. Complaints of blurred vision were higher with brinzolamide (5–6%) than dorzolamide (1%) or timolol (0%).
A meta-analysis of randomized clinical trials reported peak ocular hypotensive effect on IOP of 17% (19% to 15%) and trough effect of 17% (19% to 15%).
Side effects
Both brinzolamide and dorzolamide exhibit similar taste abnormalities. A single case report of the development of metabolic acidosis from topical brinzolamide has been described after twice-daily dosing. Other adverse events are negligible for brinzolamide except for some blurring of vision, attributable to its suspension vehicle.
Precautions
Brinzolamide has the same contraindications and precautions as dorzolamide.
Brinzolamide Preparation Products And Raw materials
Raw materials
1of4
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Siddhivinayakchemicals | +91-912225190111 +91-9819773074 | Mumbai, India | 42 | 58 | Inquiry |
| Anant Pharmaceuticals Pvt Ltd | +91-8550986868 +91-9485998001 | Haryana, India | 461 | 58 | Inquiry |
| ChemBioReas Life Sciences | +91-7989264158 +91-7989264158 | Hyderabad, India | 82 | 58 | Inquiry |
| Flax Laboratories | +91-9867665132 +91-9867665132 | Maharashtra, India | 44 | 58 | Inquiry |
| Darsh PharmaChem Pvt Ltd | +91-9712983850 +91-9712983850 | Gujarat, India | 33 | 58 | Inquiry |
| USV Pvt Ltd | +91-2267861111 +91-2225564048 | Maharashtra, India | 36 | 58 | Inquiry |
| FDC Limited | +912226782653 | Mumbai, India | 30 | 58 | Inquiry |
| Ralington Pharma | +91-7948911722 +91-9687771722 | Gujarat, India | 1350 | 58 | Inquiry |
| BDR Pharmaceuticals International Pvt Ltd | +91-2240560560 +91-7718884418 | Maharashtra, India | 206 | 58 | Inquiry |
| SMS Lifesciences India Ltd (MAHI Drugs Pvt Ltd) | +91-40-66288888 +91-8374455507 | Telangana, India | 23 | 58 | Inquiry |
138890-62-7(Brinzolamide)Related Search:
1of4
chevron_right




