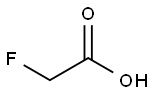
Fluoroacetic acid
- CAS No.
- 144-49-0
- Chemical Name:
- Fluoroacetic acid
- Synonyms
- MFA;HFA;2-Fluoroacetic acid;Cymonic acid;fluoroacetic;2-fluoroaceticacid;Acetic acid, 2-fluoro-;C06108;Un 2642;CH2FCOOH
- CBNumber:
- CB6212623
- Molecular Formula:
- C2H3FO2
- Molecular Weight:
- 78.04
- MOL File:
- 144-49-0.mol
- Modify Date:
- 2024/3/14 15:18:27
| Melting point | 33°C |
|---|---|
| Boiling point | 165°C |
| Density | 1.3693 |
| pka | 2.6(at 25℃) |
| Water Solubility | 50mg/L(20 ºC) |
| CAS DataBase Reference | 144-49-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Acetic acid, fluoro-(144-49-0) |
| EPA Substance Registry System | Fluoroacetic acid (144-49-0) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS06,GHS09 |
|---|---|
| Signal word | Danger |
| Hazard statements | H300-H400 |
| Precautionary statements | P264-P270-P301+P310-P321-P330-P405-P501-P273-P391-P501 |
| Hazard Codes | T+,N |
| Risk Statements | 20/21/22-35-50-28 |
| Safety Statements | 26-36/37/39-45-61-22-20 |
| RIDADR | 2642 |
| HazardClass | 6.1(a) |
| PackingGroup | I |
| Toxicity | LD50 oral in rat: 4680ug/kg |
Fluoroacetic acid Chemical Properties,Uses,Production
Chemical Properties
Fluoroacetic acid is a colorless crystalline solid.
Uses
Fluoroacetic acid (CH2FCOOH) is very poisonous. It is used to kill rats and mice.
Definition
ChEBI: A haloacetic acid that is acetic acid in which one of the methyl hydrogens is substituted by fluorine.
General Description
A colorless crystalline solid. May be toxic by ingestion. Used to make other chemicals.
Air & Water Reactions
Water soluble.
Hazard
Toxic by ingestion.
Health Hazard
Fluoroacetic acid is very toxic; ingestion of small quantities may cause death.
Fire Hazard
When heated to decomposition, Fluoroacetic acid emits highly toxic fumes of fluorine containing compounds. Some of these materials may burn but none ignite readily. These materials may ignite combustibles (wood, paper, oil, etc.).
Safety Profile
Poison by ingestion, subcutaneous, intraperitoneal, and intravenous routes. Affects the human central nervous system, causing convulsions and ventricular fibrdlation. When heated to decomposition it emits toxic fumes of F and Na2O. See also SODIUM FLUOROACETATE.
Potential Exposure
This material is used as a rodenticide and a drug.
Shipping
UN2642 Fluoroacetic acid, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explo sions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Reacts with reducing agents releasing flammable gas.
Waste Disposal
Use a licensed professional waste disposal service to dispose of this material. Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regula tions must be observed.





