Micafungin
- CAS No.
- 235114-32-6
- Chemical Name:
- Micafungin
- Synonyms
- Micfungin;MICAFUNGIN;Micafungin Na;Micafungin-d11;Micafungin (Acid);PubChem ID: 477468;PubChem ID: 3081921;PubChem ID: 11804463;PubChem ID: 78357831;Micafungin USP/EP/BP
- CBNumber:
- CB71471028
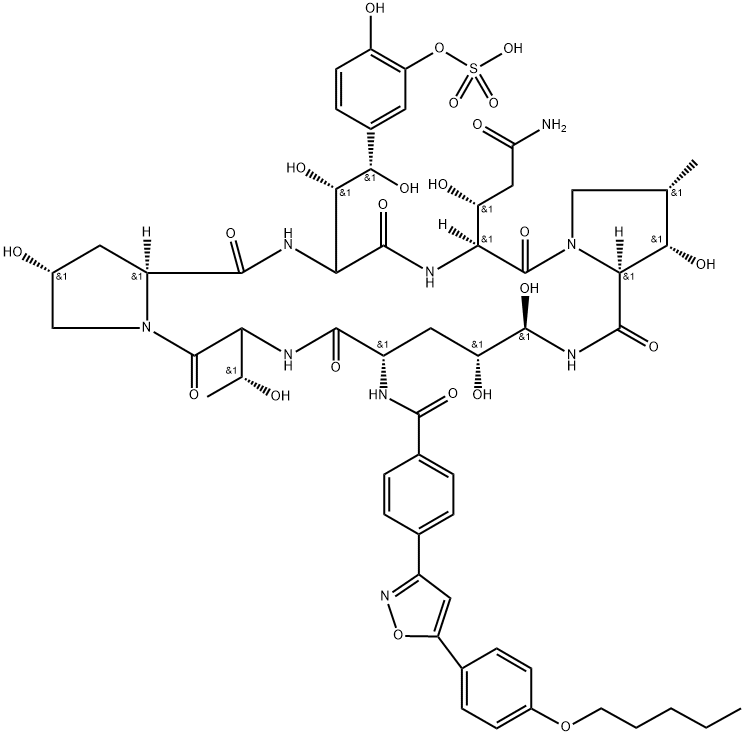
- Molecular Formula:
- C56H71N9O23S
- Molecular Weight:
- 1270.28
- MOL File:
- 235114-32-6.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/9/6 17:45:48
Micafungin Chemical Properties,Uses,Production
Description
Micafungin, the second member of the echinocandin class of antifungal agents was launched in Japan for the parenteral treatment of various fungal infections caused by Aspergillus and Candida spp. such as fungaemia and respiratory and gastrointestinal mycoses. This water-soluble semisynthetic cyclic lipopeptide is synthesized by acylation with (5-(4- pentyloxyphenyl)isoxazol-3-yl)benzoate of the cyclic peptide nucleus (FR-179642).obtained by enzymatic cleavage of the naturally occurring echinocandin FR-901379, derived from the fungus Coleophoma empedri Micafungin acts by inhibiting the synthesis of 1,3-beta-glucan, an essential polysaccharide of the cell wall of many pathogenic fungi. Micafungin has a marked fungicidal effect on almost all species of Candida, including fluconazole-resistant spp. C. albicans, C. glabrata, C. Krusei, C. parapsilosis and C. tropicalis and a fungistatic effect on a range of Aspergillus species including A. flaws, A. fumigates and A. terreus. Like caspofungin, micafungin is inactive against Cryptococcus neoformans, and the emerging pathogen Trichosporon cutaneum and Fusarium solani. Micafungin has proved highly effective in mouse models of Cancfidiasis and Aspergillus infections (including those using an amphotericin B- and itraconazole-resistant isolate of A. fumigatus). In phase I studies, micafungin had linear pharmacokinetics with an elimination half-life ranging from 11.7 to 15.2 h after injection and was well tolerated.
Uses
Micafungin is a semi-synthetic cyclic lipopeptide belonging to the echinocandin class that was reported in 1999 from Fujisawa in Japan. Unlike other marketed semi-synthetic derivatives in this class, micafungin is not derived from echinocandin but rather from FR901379 which contains a phenolic sulphate to enhance aqueous solubility, a serious limitation in the class. Micafungin inhibits the synthesis of β-(1,3)-D-glucan, an essential component of the cell wall of susceptible fungi and is extensively referenced in the literature with over 700 citations.
Definition
ChEBI: A cyclic hexapeptide echinocandin antibiotic which exerts its effect by inhibiting the synthesis of 1,3-beta-D-glucan, an integral component of the fungal cell wall. It is used as the sodium salt for the treatment of invas ve candidiasis, and of aspergillosis in patients who are intolerant of other therapy.
Antimicrobial activity
It is active against Aspergillus spp., Candida spp. and the cyst form of Pn. jirovecii. Resistance has rarely been reported.
Pharmaceutical Applications
A semisynthetic lipopeptide derived from a fermentation product of Coleophoma empetri. Formulated as the monosodium salt for intravenous infusion.
Pharmacokinetics
Cmax 50 mg 1-h infusion: c. 5 mg/L 1 h post infusion
Plasma half-life: 11–15 h
Volume of distribution: 0.4 L/kg
Plasma protein binding: 99%
Blood concentrations increase in proportion to dosage. Unlike anidulafungin and caspofungin, a loading dose is not required.
Distribution
The drug is widely distributed, the highest concentrations being found in the liver. Levels in the CSF and urine are negligible.
Metabolism and excretion
It is metabolized by the liver and the three inactive metabolites are excreted in the feces (70%). Less than 1% of a dose is eliminated as unchanged drug in the urine. No dosage adjustment is required in patients with severe renal impairment or mild to moderate hepatic impairment. The effect of severe hepatic impairment on micafungin pharmacokinetics has not been studied. Micafungin is not cleared by hemodialysis.
Clinical Use
Candidemia and certain invasive forms of candidosis
Esophageal candidosis
Prophylaxis of Candida infections in hematopoietic stem cell transplant
(HSCT) recipients
Side effects
Occasional histamine-mediated infusion-related reactions, injection site reactions and transient abnormalities of liver enzymes have been reported. Isolated cases of significant hepatic or renal dysfunction, hepatitis, or liver or renal failure have also been described.
Micafungin Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Gufic Group | +91-2267261000 +91-2267261000 | Maharashtra, India | 22 | 58 | Inquiry |
| Ralington Pharma | +91-7948911722 +91-9687771722 | Gujarat, India | 1350 | 58 | Inquiry |
| PROTECH TELELINKS | +91-8571891912 +91-9855060837 | Himachal Pradesh, India | 62 | 58 | Inquiry |
| Sumar Biotech LLP | +91-7984183709 +91-9824575381 | Gujarat, India | 22 | 58 | Inquiry |
| BDR Pharmaceuticals International Pvt Ltd | +91-2240560560 +91-7718884418 | Maharashtra, India | 206 | 58 | Inquiry |
| Apicore Pharmaceuticals Pvt Ltd | +91-2662267177 +91-2662267166 | Gujarat, India | 181 | 58 | Inquiry |
| Zydus Lifesciences Ltd. | +91-0265-2315243 +91-6358895661 | Gujarat, India | 89 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6257 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6739 | 58 | Inquiry |
| SynZeal Research Pvt Ltd | +1 226-802-2078 | Gujarat, India | 6514 | 58 | Inquiry |





