Efavirenz
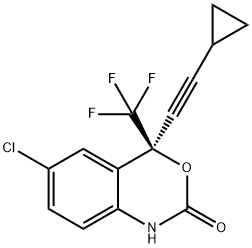
- CAS No.
- 154598-52-4
- Chemical Name:
- Efavirenz
- Synonyms
- EFV;SUSTIVA;Antiretroviral;MDP-266;DMP 266;Stocrin;L-743726;Efeveren;EFAVIRENZ;EFAVIRNEZ
- CBNumber:
- CB7181559
- Molecular Formula:
- C14H9ClF3NO2
- Molecular Weight:
- 315.67
- MOL File:
- 154598-52-4.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/5/7 13:47:35
| Melting point | 139-141°C |
|---|---|
| Boiling point | 340.6±42.0 °C(Predicted) |
| alpha | D20 -84.7° (c = 0.005 g/ml in CH3Cl); D25 -94.1° (c = 0.300 in methanol) |
| Density | 1.53±0.1 g/cm3(Predicted) |
| Flash point | 2℃ |
| storage temp. | -20°C |
| solubility | DMSO: soluble15mg/mL, clear |
| pka | 10.2(at 25℃) |
| form | powder or crystals |
| color | white to beige |
| optical activity | [α]/D -90 to -100°, c = 1 in methanol |
| Water Solubility | 8mg/L(temperature not stated) |
| λmax | 247nm(MeOH)(lit.) |
| Merck | 14,3521 |
| BCS Class | 4 |
| EPA Substance Registry System | 2H-3,1-Benzoxazin-2-one, 6-chloro-4-(2-cyclopropylethynyl)-1,4-dihydro-4-(trifluoromethyl)-, (4S)- (154598-52-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS07,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H302-H360-H410 | |||||||||
| Precautionary statements | P202-P264-P270-P273-P301+P312-P308+P313 | |||||||||
| Hazard Codes | N | |||||||||
| Risk Statements | 50 | |||||||||
| Safety Statements | 61 | |||||||||
| RIDADR | UN3082 - class 9 - PG 3 - DOT NA1993 - Environmentally hazardous substances, liquid, n.o.s. HI: all (not BR) | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | DM3440000 | |||||||||
| HS Code | 2934990002 | |||||||||
| NFPA 704 |
|
Efavirenz price More Price(4)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | SML0536 | Efavirenz ≥98% (HPLC) | 154598-52-4 | 10MG | ₹15403.98 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | SML1284 | Efavirenz Ready Made Solution 10?mg/mL in DMSO | 154598-52-4 | 1ML | ₹17236.8 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | SML0536 | Efavirenz ≥98% (HPLC) | 154598-52-4 | 50MG | ₹62633.45 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | PHR2072 | Efavirenz Pharmaceutical Secondary Standard; Certified Reference Material | 154598-52-4 | 500MG | ₹29996.08 | 2022-06-14 | Buy |
Efavirenz Chemical Properties,Uses,Production
Description
Efavirenz D5 was launched as Sustiva in the US for the treatment of infection by HIV, the virus causing AIDS, in combination with other anti-retroviral agents.
Efavirenz D5 is a non-nucleoside reverse transcriptase inhibitor (NNRTI) belonging to the 3,1-benzoxazin-2-one chemical class. It is the third non-nucleoside reverse transcriptase inhibitor to have been launched to date, after Nevirapine (1996) and Delavirdine (1997), increasing the arsenal of anti-HIV drugs for treating infected patients in dual or triple combination with nucleoside or other non-nucleoside RTIs, or protease inhibitors.
Efavirenz D5 can be obtained by two related ways of six steps from 4-chloroaniline ; one of them is based on asymmetric synthesis by enantioselective addition of an acetylide to a trifluoroacetophenone. The anti-HIV activity of Efavirenz D5 was demonstrated against most wild-type and clinical strains of HIV-1, including those with the most frequently observed mutations. Efavirenz D5 has a better pharmacokinetic profile when compared with the preceding drugs of this class ; in particular, in a long-term experiment conducted in cynomolgus monkeys, Efavirenz D5 was shown to easily cross the blood brain barrier leading to an increase of the antiviral concentration in cerebrospinal fluid.
Chemical Properties
White to Slightly Pink Crystalline Powder
Uses
Efavirenz D5 is a nonnucleoside HIV-1 reverse transcriptase inhibitor. Antiviral
Indications
Efavirenz (Sustiva) is approved for the therapy of HIV infection of adults and children and is also used for postexposure prophylaxis. It is the only NNRTI approved for once-daily dosing. Rash, although rarely severe, is a common adverse effect of efavirenz. Elevated liver enzymes and serum cholesterol also may occur. Central nervous system (CNS) effects in approximately half of patients may include dizziness, headache, insomnia, drowsiness, euphoria, agitation, impaired cognition, nightmares, vivid dreams, and hallucinations. These effects often subside after several weeks to months of therapy.
Definition
ChEBI: 1,4-Dihydro-2H-3,1-benzoxazin-2-one substituted at the 4 position by cyclopropylethynyl and trifluoromethyl groups (S configuration) and at the 6 position by chlorine. A non-nucleoside reverse transcriptase inhibitor wit activity against HIV, it is used with other antiretrovirals for combination therapy of HIV infection.
Acquired resistance
One or more single-codon substitutions in the HIV reverse transcriptase genome at positions 100, 103, 106, 108, 181, 188, 190 and 225 confer reduced susceptibility. Many, but not all, of these point mutations confer reduced susceptibility to other non-nucleoside reverse transcriptase inhibitors.
General Description
Efavirenz D5 (Sustiva)84 is also mandated for use with at leasttwo other antiretroviral agents. The compound is morethan 99% protein bound, and CSF concentrations exceedthe free fraction in the serum. Metabolism occurs in theliver. The half-life of a single dose of Efavirenz D5 is 52 to 76hours, and 40 to 55 after multiple doses (the drug inducesits own metabolism). Peak concentration is achieved in 3to 8 hours. Elimination is 14% to 34% in urine (as metabolites)and 16% to 41% in feces (primarily as Efavirenz D5).The oral dosage form is supplied as a capsule.
Pharmaceutical Applications
Efavirenz D5 is a synthetic heterocyclic compound formulated for oral administration.
Pharmacology
Efavirenz interacts with many drugs via the cytochrome P450 pathways. It induces and is metabolized by CYP3A4 and inhibits CYP2C9 and CYP2C19. It should not be given with cisapride, ergot alkaloids, midazolam, or triazolam because of the potential for lifethreatening reactions. Efavirenz has the potential to decrease blood levels of methadone, rifabutin, ketoconazole, and itraconazole. It may inhibit the metabolism of drugs such as alosetron, diazepam, ethinyl estradiol, imipramine, losartan, omeprazole, warfarin, tolbutamide, and topiramate. Efavirenz interacts with cytochrome P450 inducers and substrates (e.g., phenytoin, phenobarbital) in a complex manner; blood levels and side effects should be closely monitored. Patients taking efavirenz should avoid herbal preparations containing St. John’s wort because the herb induces CYP3A4 and may cause drug failure or viral resistance. Saquinavir should not be used as the sole protease inhibitor in a regimen containing efavirenz.
Pharmacokinetics
Oral absorption: Not known/available
Cmax 600 mg oral once daily: c. 4.07 mg/L
Cmin 600 mg oral once daily: c. 1.77 mg/L
Plasma half-life: c. 45 h
Volume of distribution: c. 2.4 L/kg
Plasma protein binding: >99%
Absorption and distribution
Bioavailability following a standard high-fat meal was increased by an average of 50%, but was unaffected by a standard meal. Distribution into body tissues and fluids has not been fully characterized. It penetrates moderately well into the CNS. The semen:plasma ratio is 0.09 (0.03–0.43). The mean concentration in breast milk is 3.51 mg/L; significant linear correlations have been found between maternal plasma and breast milk.
Metabolism and excretion
It is metabolized by cytochrome P450 systems to hydroxylated intermediates and excreted after subsequent glucuronidation. Metabolites are not active against HIV.
It is excreted principally in the feces, both as metabolites and unchanged drug. Up to 34% is recovered in the urine, <1% as unchanged drug. Given this, the impact of renal impairment on efavirenz is likely to be minimal. Caution is recommended in patients with mild–moderate liver disease; it is contraindicated in patients with severe hepatic impairment.
Dose adjustment is unnecessary when it is co-administered with HIV protease inhibitors or rifampicin (rifampin).
Clinical Use
Treatment of HIV-1 infection in adults and children (in combination with other antiretroviral drugs)
Side effects
The most common (>5%, moderate–severe) adverse effects associated with Efavirenz D5 therapy are rash, dizziness, nausea, headache, fatigue, insomnia and vomiting. Rash occurs in up to 26% of patients, mostly in the first 2 weeks of therapy. It usually resolves within 1 month, but is sufficiently severe to limit treatment in a few cases.
Dizziness, insomnia, somnolence, impaired concentration, abnormal dreaming and other CNS disturbances have been reported in around 52% of clinical trial participants, with events of moderate to severe intensity occurring in about 3% of patients. Rare (0.2% of patients) episodes of severe delusional or inappropriate behavior and severe acute depression have also been reported. The symptoms commonly begin in the first 2 weeks of treatment but often resolve or substantially improve within a month.
Elevations in serum hepatic transaminase to levels more than five times the upper limit of normal are observed in about 3% of patients and 8% of those co-infected with viral hepatitis B or C.
Efavirenz Preparation Products And Raw materials
Raw materials
1of4
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| J S LABS | +91-7330612784 +91-7330612784 | Tamil Nadu, India | 160 | 58 | Inquiry |
| Aurobindo Pharma Limited | +914066725000 | Telangana, India | 112 | 58 | Inquiry |
| Apotex Pharmachem India Pvt Ltd | +91-8022891034 +91-8022891000 | Karnataka, India | 109 | 58 | Inquiry |
| Cipla Ltd | +912224826000 | Maharashtra, India | 133 | 58 | Inquiry |
| Hetero Drugs Limited | +91-4023704923 +91-4023704923 | Telangana, India | 296 | 58 | Inquiry |
| Chorus Labs Limited | +91-9640678999 +91-9640678999 | Hyderabad, India | 11 | 58 | Inquiry |
| HRV Global Life Sciences | +91-9820219686 +91-9820219686 | Telangana, India | 379 | 58 | Inquiry |
| Mangalam Drugs and Organics Ltd | +91-2222616300 +91-2222616200 | Maharashtra, India | 37 | 58 | Inquiry |
| Chempifine Chemicals | +91-2225667766 +91-2225667766 | Punjab, India | 144 | 58 | Inquiry |
| VIVIN DRUGS AND PHARMACEUTICALS LTD | +91-22670456625 +91-224067045635 | Telangana, India | 22 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| J S LABS | 58 |
| Aurobindo Pharma Limited | 58 |
| Apotex Pharmachem India Pvt Ltd | 58 |
| Cipla Ltd | 58 |
| Hetero Drugs Limited | 58 |
| Chorus Labs Limited | 58 |
| HRV Global Life Sciences | 58 |
| Mangalam Drugs and Organics Ltd | 58 |
| Chempifine Chemicals | 58 |
| VIVIN DRUGS AND PHARMACEUTICALS LTD | 58 |
Related articles
- Efavirenz---A first-line agent for HIV-1 infection
- Efavirenz, earlier known as L-743,726 and DMP-266, is a benzoxazinone compound that was developed by DuPont Merck at Merck Res....
- Apr 19,2022
154598-52-4(Efavirenz)Related Search:
1of4
chevron_right




