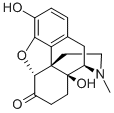
OXYMORPHONE
- CAS No.
- 76-41-5
- Chemical Name:
- OXYMORPHONE
- Synonyms
- NSC 19045;NIH 10323;Numorphan;Oxymorphine;OXYMORPHONE;Oxymorphone (CRM);Dihydroxymorphinone;Naltrexone Impurity 15;OXYMORPHONE (MW 301.35);Dihydrohydroxymorphinone
- CBNumber:
- CB8317944
- Molecular Formula:
- C17H19NO4
- Molecular Weight:
- 301.34
- MOL File:
- 76-41-5.mol
- Modify Date:
- 2023/6/8 9:03:07
| Melting point | 248-249°C (dec) |
|---|---|
| Boiling point | 518.6±50.0 °C(Predicted) |
| Density | 1.50±0.1 g/cm3(Predicted) |
| Flash point | 9℃ |
| storage temp. | Controlled Substance, -20°C Freezer |
| solubility | Soluble in DMSO |
| form | Powder |
| pka | pKa 8.50 (Uncertain);9.33 (Uncertain) |
| EPA Substance Registry System | Morphinan-6-one, 4,5-epoxy-3,14-dihydroxy-17-methyl-, (5.alpha.)- (76-41-5) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302-H336 | |||||||||
| Precautionary statements | P301+P312+P330 | |||||||||
| Hazard Codes | F,T,T+ | |||||||||
| Risk Statements | 11-23/24/25-39/23/24/25-26/27/28 | |||||||||
| Safety Statements | 16-36/37-45-36/37/39-22 | |||||||||
| RIDADR | UN1230 - class 3 - PG 2 - Methanol, solution | |||||||||
| WGK Germany | 1 | |||||||||
| HS Code | 2939110000 | |||||||||
| NFPA 704 |
|
OXYMORPHONE Chemical Properties,Uses,Production
Chemical Properties
Crystalline Solid
Uses
Controlled substance (opiate). Analgesic (narcotic)
Biological Functions
Oxymorphone is 10 times as potent as morphine, with actions similar to those of hydromorphone. Oxymorphone, however, has little antitussive activity, and as such is a useful analgesic in patients with pulmonary disease who need to retain the ability to cough.
General Description
Oxymorphone is the 14 beta-hydroxyl version of hydromorphone,analogous to the hydrocodone, oxycodone pair discussedabove. Although the addition of the 14 beta-hydroxylgroup to hydrocodone (30 mg) yielded oxycodone (20 mg),a more potent drug, the opposite is true for the conversion ofhydromorphone (7.5 mg) to oxymorphone (10 mg). The reasonfor this is that the oral bioavailability of oxymorphone(10%) is lower than that of hydromorphone (35%) becauseof decreased absorption and increased first-pass metabolism.Presumably, the addition of the OH group does increaseits binding affinity at the receptor as the injectableform of oxymorphone (1 mg) is more potent than injectablehydromorphone (1.5 mg).
Oxymorphone is available as a suppository (5 mg), an injection(1 mg/mL), an immediate-release tablet (5 mg, 10mg), and in 2003 the FDA approved a sustained release formulation(Opana ER 5 mg, 10 mg, 20 mg, and 40 mg). The12-hour coverage of the extended release tablet provides anotheroption for those patients suffering from chronic pain.The side effect profile of the extended release formulationsof morphine, oxycodone, and oxymorphone are similar, andthere appears to be no clear advantage of one over the other.
OXYMORPHONE Preparation Products And Raw materials
Raw materials
Preparation Products
76-41-5(OXYMORPHONE)Related Search:
1of4
chevron_right




