TOFOGLIFLOZIN
- CAS No.
- 1201913-82-7
- Chemical Name:
- TOFOGLIFLOZIN
- Synonyms
- Tofogliflozin Monohydrate;CSG452; CSG-452; TOFOGLIFLOZIN; CSG 452; UNII-554245W62TTOFOGLIFLOZIN [INN]; TOFOGLIFLOZIN ANYHYDROUS;Togliflozin;CSG 452(hydrate);Tofogliflozin(CSG452);Tofogliflozin (hydrate);TOFOGLIFLOZIN USP/EP/BP;Tofogliflozin anyhydrous;Tofogliflozin Hydrate (JAN);Tofogliflozin hydrate (1:1)
- CBNumber:
- CB92667680
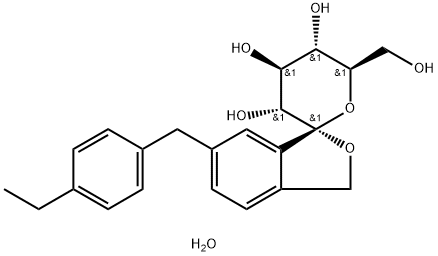
- Molecular Formula:
- C22H26O6.H2O
- Molecular Weight:
- 404.46
- MOL File:
- 1201913-82-7.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/7/2 8:55:41
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302-H315-H319-H335 | |||||||||
| Precautionary statements | P261-P305+P351+P338 | |||||||||
| NFPA 704 |
|
TOFOGLIFLOZIN Chemical Properties,Uses,Production
Description
Tofogliflozin hydrate, which is a sodium-glucose co-transporter 2 inhibitor, was approved in Japan for the treatment of type 2 diabetes at the same time as luseogliflozin hydrate (XIX). The drug was discovered by Chugai Pharmaceutical and jointly developed with Sanofi-Aventis and Kowa. Tofogliflozin hydrate reduces glucose levels by inhibiting the reuptake of glucose by selectively inhibiting SGLT2, and plays a key role in the reuptake of glucose in the proximal tubule of the kidneys.
Synthesis
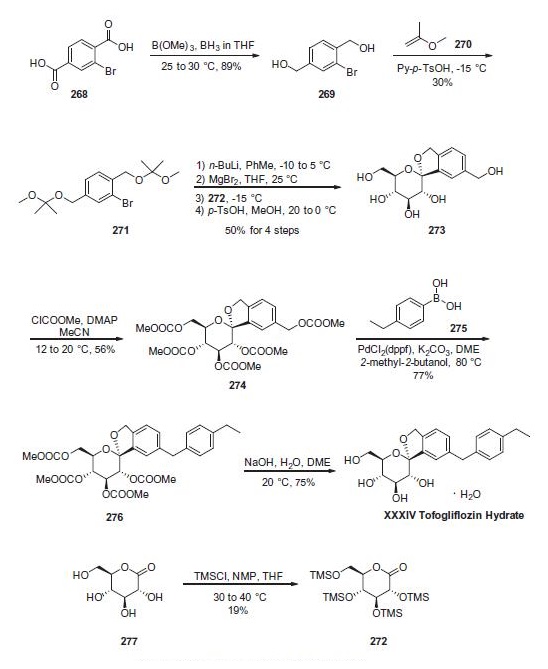
Reduction of commercially available 2-bromoterephtalic acid (268) through the use of trimethoxyborane and borane- THF proceeded in 89% yield to afford diol 269. Subjection of this compound to 2-methoxypropene (270) under acidic conditions generated bis-acetonide 271. This bromide then underwent lithium¨Chalogen exchange followed by exposure to magnesium bromide and treatment with lactone 272 (which was prepared by persilylation of commercially available (3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2Hpyran- 2-one (277). This mixture was worked up with aqueous ammonium chloride and upon treatment with p-TsOH in methanol resulted in spiroacetal 273. Next, global protection of all alcohol functionalities within 273 was affected by reaction with methylchloroformate and DMAP in acetonitrile. The benzyl carbonate within 274 was selectively exchanged via Suzuki coupling with 4-ethylphenylboronic acid (275) to afford methylene dibenzyl system 276. Subsequent treatment with aqueous sodium hydroxide in methanol followed by crystallization from 1:6 acetone and water furnished the desired product tofogliflozin hydrate (XXXIV) in 75% yield.
TOFOGLIFLOZIN Preparation Products And Raw materials
Raw materials
Preparation Products
TOFOGLIFLOZIN Suppliers
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| BOC Sciences | +1-631-485-4226 | United States | 19553 | 58 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29826 | 58 | Inquiry |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | China | 9030 | 58 | Inquiry |
| AFINE CHEMICALS LIMITED | +86-0571-85134551 | China | 15395 | 58 | Inquiry |
| InvivoChem | +1-708-310-1919 +1-13798911105 | United States | 6393 | 58 | Inquiry |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418684 +8618949823763 | China | 25363 | 58 | Inquiry |
| LEAP CHEM CO., LTD. | +86-852-30606658 | China | 24738 | 58 | Inquiry |
| TargetMol Chemicals Inc. | +1-781-999-5354 | United States | 19973 | 58 | Inquiry |
| SUZHOU SENFEIDA CHEMICAL CO.,LTD | +86-0512-83500002 +8615195660023 | China | 23053 | 58 | Inquiry |
| Shanghai Qianyi New Material Co., Ltd. | +8618083322317 | China | 878 | 58 | Inquiry |
1201913-82-7(TOFOGLIFLOZIN)Related Search:
1of4
chevron_right




