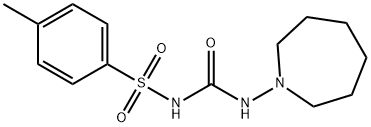
TOLAZAMIDE
- CAS No.
- 1156-19-0
- Chemical Name:
- TOLAZAMIDE
- Synonyms
- U-17835;diabewas;tolanase;tolinase;Tolonase;norglycin;nsc-70762;olazamide;nci-c03327;TOLAZAMIDE
- CBNumber:
- CB9355686
- Molecular Formula:
- C14H21N3O3S
- Molecular Weight:
- 311.4
- MOL File:
- 1156-19-0.mol
- Modify Date:
- 2023/6/8 9:02:53
| Melting point | 162-164°C |
|---|---|
| Boiling point | 300°C (rough estimate) |
| Density | 1.2228 (rough estimate) |
| refractive index | 1.6740 (estimate) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Very slightly soluble in water; freely soluble in chloroform; soluble in acetone; slightly soluble in ethanol (96%). |
| form | Solid |
| pka | 3.6(at 25℃) |
| color | White to Off-White |
| Water Solubility | 65.4mg/L(30 ºC) |
| CAS DataBase Reference | 1156-19-0(CAS DataBase Reference) |
| EPA Substance Registry System | Tolazamide (1156-19-0) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H370 | |||||||||
| Precautionary statements | P260-P264-P270-P307+P311-P321-P405-P501 | |||||||||
| Hazard Codes | Xn | |||||||||
| Risk Statements | 22 | |||||||||
| Safety Statements | 36 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | YT4400000 | |||||||||
| HS Code | 2935904000 | |||||||||
| Toxicity | LD50 in rats, mice (mg/kg): >5000 orally, 2239 i.p. (Dulin) | |||||||||
| NFPA 704 |
|
TOLAZAMIDE Chemical Properties,Uses,Production
Description
Tolazamide is a first generation sulfonylurea that inhibits sulfonylurea receptor 1 (SUR1) linked to the inwardly rectifying potassium channel (KIR6.2; IC50 = 4.2 μM in HEK293 cells transfected with the human receptor). It has no effect on glucose uptake in L6 rat skeletal muscle cells when used at a concentration of 0.6 mg/mL but enhances glucose uptake two-fold when used in combination with insulin. In vivo, tolazamide (128 mg/kg) reduces glomerulosclerosis and albumin excretion in a rat model of insulin-dependent diabetes induced by streptozotocin . Formulations containing tolazamide have been used in the treatment of type 2 diabetes.
Chemical Properties
White Solid
Uses
Labelled Tolazamide, an antidiabetic.
Definition
ChEBI: An N-sulfonylurea that is 1-tosylurea in which a hydrogen attached to the nitrogen at position 3 is replaced by an azepan-1-yl group. A hypoglycemic agent, it is used for the treatment of type 2 diabetes mellitus.
General Description
Tolazamide is N-[[(hexahydro-1H-azepin-1-yl)amino]carbonyl]-4-methylbenzenesulfonamide; or 1-(hexahydro-1H-azepin-1-yl)-3-(p-tolylsulfonyl)urea; or 1-(4-methylphenylsulfonyl)-3-(hexahydro-1H-azepin-1-yl)urea (generic).Tolazamide incorporates a fully saturated azepine moietythat is but weakly basic, with a pKa of~3.32 The pKa of thesulfonylurea group lies within the typical range; thus, inareas of the duodenum wherein the pH falls within the rangeof 4 to 5, the uncharged form of the drug is the predominantspecies, and its lipophilicity lends to rapid absorption bypassive diffusion.
Air & Water Reactions
TOLAZAMIDE may be sensitive to prolonged exposure to air. Insoluble in water.
Reactivity Profile
TOLAZAMIDE is an amide. Amides/imides react with azo and diazo compounds to generate toxic gases. Flammable gases are formed by the reaction of organic amides/imides with strong reducing agents. Amides are very weak bases (weaker than water). Imides are less basic yet and in fact react with strong bases to form salts. That is, they can react as acids. Mixing amides with dehydrating agents such as P2O5 or SOCl2 generates the corresponding nitrile. The combustion of these compounds generates mixed oxides of nitrogen (NOx). TOLAZAMIDE is incompatible with acids. .
Fire Hazard
Flash point data for TOLAZAMIDE are not available; however, TOLAZAMIDE is probably combustible.
TOLAZAMIDE Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| Triveni chemicals | 08048762458 | New Delhi, India | 6093 | 58 | Inquiry |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | China | 32760 | 60 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29826 | 58 | Inquiry |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | United States | 19892 | 58 | Inquiry |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | China | 34571 | 58 | Inquiry |
| Finetech Industry Limited | +86-27-87465837 +8618971612321 | China | 9624 | 58 | Inquiry |
| Zhejiang J&C Biological Technology Co.,Limited | +1-2135480471 +1-2135480471 | China | 10522 | 58 | Inquiry |
1156-19-0(TOLAZAMIDE)Related Search:
1of4
chevron_right




