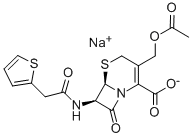
Cephalothin sodium
- CAS No.
- 58-71-9
- Chemical Name:
- Cephalothin sodium
- Synonyms
- 38253;Coaxin;Keflin;Seffin;Averon 1;Cemastin;Lospoven;Microtin;synclotin;Cephation
- CBNumber:
- CB9443527
- Molecular Formula:
- C16H16N2O6S2.Na
- Molecular Weight:
- 418.42
- MOL File:
- 58-71-9.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/7/2 8:55:42
| Melting point | 240°C |
|---|---|
| alpha | D +135° (c = 1.0 in water) |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | H2O: 50 mg/mL, clear, faintly yellow |
| form | Solid |
| color | White to Off-White |
| Water Solubility | 158 mg/L |
| Merck | 13,1994 |
| BRN | 4120706 |
| Stability | Hygroscopic |
| CAS DataBase Reference | 58-71-9(CAS DataBase Reference) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|---|---|
| Signal word | Danger |
| Hazard statements | H317-H334 |
| Precautionary statements | P261-P280-P284-P304+P340-P342+P311 |
| Hazard Codes | Xn,Xi |
| Risk Statements | 42/43 |
| Safety Statements | 22-36/37 |
| WGK Germany | 2 |
| RTECS | XI0388300 |
| HS Code | 29419000 |
| Toxicity | LD50 in mice, rats (mg/kg): >20000, >10000 orally; 5670, 7716 i.p. (Kuramoto) |
Cephalothin sodium price More Price(6)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | C4520 | Cephalothin sodium salt | 58-71-9 | 100MG | ₹2890.28 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | C4520 | Cephalothin sodium salt | 58-71-9 | 250MG | ₹3377.4 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | C4520 | Cephalothin sodium salt | 58-71-9 | 1G | ₹7739.88 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | C4520 | Cephalothin sodium salt | 58-71-9 | 5G | ₹26348.05 | 2022-06-14 | Buy |
| SRL | 36413 | Cephalothin Sodium Salt (CF), 97% | 58-71-9 | 1Gms | ₹3170 | 2022-05-26 | Buy |
Cephalothin sodium Chemical Properties,Uses,Production
Description
Cefalothin is a β-lactam cephalosporin antibiotic. It inhibits the growth of various Gram-positive and Gram-negative bacteria, including several strains of S. pyogenes, S. aureus, C. tetani, N. gonorrhoeae, Salmonella, and Shigella (MICs = 0.1-0.2, 0.312-0.625, 0.078, 1.25, 1.56-6.25, and 3.12-12.5 μg/ml, respectively). Cefalothin binds to E. coli penicillin-binding proteins (PBPs; IC50s = <0.25, 16, 37, and 1 μg/ml for PBP1a, 1bs, 2, and 3, respectively, in a radioligand binding assay), which interferes with bacterial morphogenesis. It exhibits antibacterial activity in mouse models of infection with S. pyogenes, D. pneumoniae, and S. aureus. Formulations containing cefalothin were previously used in the prophylaxis and treatment of bacterial infections.
Chemical Properties
Crystalline
Uses
Cephalothin sodium salt is used to study the mechanism of liposome encapsulated antibiotics1, strategies for co-opting β-lactamases of Gram-negative bacteria for treatment of antibiotics2, and for immunology studies in relation to antibiotics.3 It is used to study the effect of expression, binding and inhibition of penicillin-binding proteins especially PBP6 on bacterial cell wall mucopeptide synthesis.
General Description
Cephalothin, along with cephaloridine, was the first of the synthetic cephalosporin C class antibiotics to be introduced clinically. It was synthesized from 7-amino-cephalosporanic acid by Lilly Research Laboratories in 1962. Cephalothin shows strong activity against gram-positive and gram-negative bacteria and Leptospira, including benzylpenicillin-resistant strains. It has been used intravenously and intramuscularly to treat a variety of infections caused by Staphylococcus, Streptococcus, Escherichia coli, and Neisseria. The drug is metabolized in vivo, and the metabolite, deacetylcephalothin, is almost inactive.
Clinical Use
Cephalothin sodium (Keflin) occurs as a white to off-white,crystalline powder that is practically odorless. It is freelysoluble in water and insoluble in most organic solvents.Although it has been described as a broad-spectrum antibacterialcompound, it is not in the same class as the tetracyclines.Its spectrum of activity is broader than that ofpenicillin G and more similar to that of ampicillin. Unlikeampicillin, cephalothin is resistant to penicillinase producedby S. aureus and provides an alternative to the use ofpenicillinase-resistant penicillins for the treatment of infectionscaused by such strains.
Cephalothin is absorbed poorly from the GI tract andmust be administered parenterally for systemic infections. It is relatively nontoxic and acid stable. It is excreted rapidlythrough the kidneys; about 60% is lost within 6 hours of administration.Pain at the site of intramuscular injection andthrombophlebitis following intravenous injection have beenreported. Hypersensitivity reactions have been observed,and there is some evidence of cross-sensitivity in patientsnoted previously to be penicillin sensitive.
Cephalothin sodium Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| GLP Pharma Standards | +91 9866074638 | Hyderabad, India | 1644 | 58 | Inquiry |
| Aavyan Labs | +91-9652399498 +91-9652399498 | Telangana, India | 60 | 58 | Inquiry |
| Aruvi Labs | +91-8056181939 +91-8056181939 | Tamil Nadu, India | 149 | 58 | Inquiry |
| Aavyan Labs | 91-9652399498 | Hyderabad, India | 19 | 58 | Inquiry |
| Anika International Pvt. Limited | 91-11-47289900 | Delhi, India | 94 | 58 | Inquiry |
| Aruvi Labs | 91-0-8056181939 | Tamil Nadu, India | 68 | 58 | Inquiry |
| Maas Pharma Chemicals | +91-9958666224 | Delhi, India | 1607 | 58 | Inquiry |
| Medkem Laboratories | 91-40-23700606 | Andhra Pradesh, India | 297 | 58 | Inquiry |
| Metro Exporters Pvt. Ltd. | 91-22-24916500 | Maharashtra, India | 204 | 58 | Inquiry |
| Anand Agencies | 91-20-24454597 | Maharashtra, India | 2337 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| GLP Pharma Standards | 58 |
| Aavyan Labs | 58 |
| Aruvi Labs | 58 |
| Aavyan Labs | 58 |
| Anika International Pvt. Limited | 58 |
| Aruvi Labs | 58 |
| Maas Pharma Chemicals | 58 |
| Medkem Laboratories | 58 |
| Metro Exporters Pvt. Ltd. | 58 |
| Anand Agencies | 58 |
58-71-9(Cephalothin sodium)Related Search:
1of4
chevron_right




