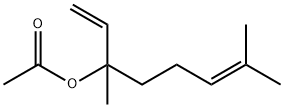
Linalyl acetate
- CAS No.
- 115-95-7
- Chemical Name:
- Linalyl acetate
- Synonyms
- LINALOOL ACETATE;LINALYL ACETATE NATURAL;Bergamol;FEMA 2636;linalyl;Linallyl Acetate;1,6-Octadien-3-ol, 3,7-dimethyl-, 3-acetate;Bergamiol;Linalylacetat;linalolacetate
- CBNumber:
- CB0674881
- Molecular Formula:
- C12H20O2
- Molecular Weight:
- 196.29
- MOL File:
- 115-95-7.mol
- MSDS File:
- SDS
- Modify Date:
- 2025/3/10 19:13:56
| Melting point | 85°C |
|---|---|
| Boiling point | 220 °C(lit.) |
| Density | 0.901 g/mL at 25 °C(lit.) |
| vapor density | 6.8 (vs air) |
| vapor pressure | 0.1 mm Hg ( 20 °C) |
| FEMA | 2636 | LINALYL ACETATE |
| refractive index |
n |
| Flash point | 194 °F |
| storage temp. | -20°C |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Liquid |
| color | Clear colorless |
| Odor | at 100.00 %. sweet green citrus bergamot lavender woody |
| Odor Type | herbal |
| optical activity | (S)-:-9.4520 |
| biological source | synthetic |
| Water Solubility | 499.8mg/L(25 ºC) |
| Merck | 14,5496 |
| JECFA Number | 359 |
| BRN | 1724500 |
| InChIKey | UWKAYLJWKGQEPM-LBPRGKRZSA-N |
| LogP | 3.9 at 25℃ |
| CAS DataBase Reference | 115-95-7(CAS DataBase Reference) |
| NIST Chemistry Reference | 1,6-Octadien-3-ol, 3,7-dimethyl-, acetate(115-95-7) |
| EPA Substance Registry System | Linalyl acetate (115-95-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H317-H319 | |||||||||
| Precautionary statements | P261-P264-P272-P280-P302+P352-P305+P351+P338 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 36/37/38-38 | |||||||||
| Safety Statements | 26-36-37-24/25 | |||||||||
| RIDADR | NA 1993 / PGIII | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | RG5910000 | |||||||||
| HS Code | 29153900 | |||||||||
| Hazardous Substances Data | 115-95-7(Hazardous Substances Data) | |||||||||
| Toxicity | LD50 orally in Rabbit: 13934 mg/kg | |||||||||
| NFPA 704 |
|
Linalyl acetate price More Price(15)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | W263613 | Linalyl acetate natural, ≥96%, FG | 115-95-7 | 1SAMPLE-K | ₹5196 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | W263613 | Linalyl acetate natural, ≥96%, FG | 115-95-7 | 100G | ₹8595.05 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | W263613 | Linalyl acetate natural, ≥96%, FG | 115-95-7 | 1KG | ₹47056.28 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | W263605 | Linalyl acetate ≥97%, FCC, FG | 115-95-7 | 1SAMPLE-K | ₹5141.88 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | W263605 | Linalyl acetate ≥97%, FCC, FG | 115-95-7 | 1KG | ₹8638.35 | 2022-06-14 | Buy |
Linalyl acetate Chemical Properties,Uses,Production
Chemical Properties
Linalyl Acetate occurs as its isomer as the main component of lavender oil (30–60%, depending on the origin of the oil), of lavandin oil (25–50%, depending on the species), and of bergamot oil (30–45%). It has also been found in clary sage oil (up to 75%) and in a small amount in many other essential oils. Racemic linalyl acetate is a colorless liquid with a distinct bergamot–lavender odor.

Linalyl acetate is used extensively in perfumery. It is an excellent fragrance material for, among others, bergamot, lilac, lavender, linden, neroli, ylang-ylang, and fantasy notes (particularly chypre). Smaller amounts are used in other citrus products. Since linalyl acetate is fairly stable toward alkali, it can also be employed in soaps and detergents.
Occurrence
Reported found in the essential oils of bergamot, lavender, clary sage and lavandin; also identified among the constituents of the essential oils of Salvia officinalis, petitgrain, sassafras, neroli, lemon, Italian lime, jasmine, Mentha citrata, Mentha aquatica, Thymus mastichina, etc; also reported in abundant quantities in the essential oil from flowers, leaves and stems of Tagetes patula, in the distillate from leaves of Citrus aurantifolia from India, and in the essential oil of Mentha arvensis. Also reported found in citrus peel oils and juices, berries, peach, celery, tomato, cinnamon, clove, nutmeg, pepper, thymus, grape wines, avocado, mushroom, marjoram, mango, cardamom, coriander, gin, origanum, lovage, laurel, myrtle leaf, rosemary, sage and mastic gum oil.
Uses
Linalyl Acetate ermentative production of medium or short chain length alcohol, esters and/or glucosides by metabolically engineered microorganism.
Preparation
Linalyl acetate is synthesized by two methods:
1) Esterification of linalool requires special reaction conditions since it tends to
undergo dehydration and cyclization as it is an unsaturated tertiary alcohol.
These reactions can be avoided as follows: esterification with ketene in the
presence of an acidic esterification catalyst below 30 °C results in the formation
of linalyl acetate without any by-products. Esterification can be achieved in good yield, with boiling acetic anhydride, whereby the acetic
acid is distilled off as it is formed; a large excess of acetic anhydride must
be maintained by continuous addition of anhydride to the still vessel.
Highly pure linalyl acetate can be obtained by transesterification of tert-butyl
acetate with linalool in the presence of sodium methylate and by continuous
removal of the tert-butanol formed in the process.
2) Dehydrolinalool, obtained by ethynylation of 6-methyl-5-hepten-2-one, can
be converted into dehydrolinalyl acetatewith acetic anhydride in the presence
of an acidic esterification catalyst. Partial hydrogenation of the triple bond
to linalyl acetate can be carried out with, for example, palladium catalysts
deactivated with lead.
General Description
Linalyl acetate is a monoterpene ester mainly found in lavandula essential oil. It is used as a flavoring agent in food industries, as well as a preservative additive in cosmetics and fragrances such as soaps, colognes, perfumes, and skin lotions.
Contact allergens
Structurally close to linalool, linalyl acetate is the main component of lavender oil and is commonly used in fragrances and toiletries, and in household cleaners and detergents as well. By autoxidation, it leads mainly to hydroperoxides, with a high sensitizing potent.
Linalyl acetate Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| JD Chem India | +91-9899117932 +91-9555260045 | New Delhi, India | 41 | 58 | Inquiry |
| Silverline Chemicals | +91-9810339289 +91-9810339289 | Haryana, India | 71 | 58 | Inquiry |
| Swati Menthol and Allied Chemicals Ltd | +91-9837848873 +91-9837848871 | Uttar Pradesh, India | 49 | 58 | Inquiry |
| Dodhia Group | +91-9619867345 +91-9619867345 | Mumbai, India | 129 | 58 | Inquiry |
| HRV Global Life Sciences | +91-9820219686 +91-9820219686 | Telangana, India | 379 | 58 | Inquiry |
| Ghaziabad Aromatics | +91-9811327736 +91-9654012346 | Uttar Pradesh, India | 80 | 58 | Inquiry |
| CHEMSWORTH | +91-261-2397244 | New Delhi, India | 6700 | 30 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6257 | 58 | Inquiry |
| TCI Chemicals (India) Pvt. Ltd. | 1800 425 7889 | New Delhi, India | 6768 | 58 | Inquiry |
| Otto Chemie Pvt. Ltd. | +91 9820041841 | Mumbai, India | 5870 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| JD Chem India | 58 |
| Silverline Chemicals | 58 |
| Swati Menthol and Allied Chemicals Ltd | 58 |
| Dodhia Group | 58 |
| HRV Global Life Sciences | 58 |
| Ghaziabad Aromatics | 58 |
| CHEMSWORTH | 30 |
| CLEARSYNTH LABS LTD. | 58 |
| TCI Chemicals (India) Pvt. Ltd. | 58 |
| Otto Chemie Pvt. Ltd. | 58 |
115-95-7(Linalyl acetate)Related Search:
1of4
chevron_right




