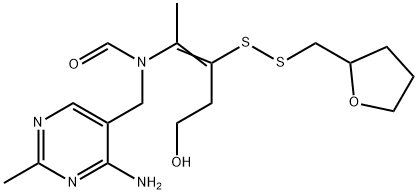
Fursultiamine
- CAS No.
- 804-30-8
- Chemical Name:
- Fursultiamine
- Synonyms
- ttfd;judolor;linamin;Retar B1;adventan;diteftin;Alinaxin;alinaminf;Aliaron F;fursultiamin
- CBNumber:
- CB1161096
- Molecular Formula:
- C17H26N4O3S2
- Molecular Weight:
- 398.54
- MOL File:
- 804-30-8.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/3/26 14:13:49
| Melting point | 130-136°C (decompos.) |
|---|---|
| Boiling point | 652.3±55.0 °C(Predicted) |
| Density | 1.29 |
| refractive index | 1.6270 (estimate) |
| storage temp. | Keep in dark place,Inert atmosphere,Store in freezer, under -20°C |
| solubility | soluble in organic solvents such as ethanol, DMSO, and dimethyl formamide (DMF). |
| form | Solid |
| pka | 14.42±0.10(Predicted) |
| color | White to Off-White |
| CAS DataBase Reference | 804-30-8(CAS DataBase Reference) |
SAFETY
Risk and Safety Statements
| Toxicity | LD50 in rats (mg/kg): 2200 orally; 540 i.p. (Yurugi, Fushimi) |
|---|
Fursultiamine Chemical Properties,Uses,Production
Description
Fursultiamine (syn. thiamine tetrahydrofurfuryl disulfide) is a vitamin B1 derivative which is rarely used, is only sparingly soluble in water and thus is usually employed only for solid drug forms. Lyophilisates are stabilized by sodium dextran sulfate.
Chemical Properties
white or slightly yellow crystalline powder. melting point 130-136 °C (decomposition). soluble in methanol, ethanol, chloroform, slightly soluble in acetone, insoluble in benzene, ether, slightly garlic odor.
Uses
Fursultiamine is an oral source of thiamine, used in comparative pharmacokinectic analysis of thiamine and it’s phosphorylated metabolites administered as multivitamin preparations, identification of drug candidate against prostate cancer from aspect of somatic cell reprogramming, therapeutic tool in number of human neurological diseases.
Application
Fursultiamine, a lipophilic vitamin B1 derivative, which is more readily absorbed in large amounts in the small intestine and has a longer half-life than its active counterpart vitamin B1. It is suitable for the treatment of beriberi or Wernicke encephalopathy due to vitamin B1 deficiency. It can also be used for the adjuvant treatment of peripheral neuritis and indigestion caused by vitamin B1 deficiency.
Preparation
Furanthiamine is obtained by condensing thiamine hydrochloride with sodium tetrahydrofuranmethyl thiosulfate after ring opening.
Fursultiamine Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Ami Lifesciences Pvt Ltd | +91-9426998100 +91-9426998100 | Gujarat, India | 62 | 58 | Inquiry |
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Shandong Natural Micron Pharm Tech Co.,LTD | +86-18653895227 | China | 94 | 58 | Inquiry |
| Wuhan Qiami Technology Co., Ltd | +8618062705058 | China | 148 | 58 | Inquiry |
| Hebei Yanxi Chemical Co., Ltd. | +86-17531190177; +8617531190177 | China | 6011 | 58 | Inquiry |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | China | 21675 | 55 | Inquiry |
| Shanghai Time Chemicals CO., Ltd. | +86-021-57951555 +8617317452075 | China | 1807 | 55 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29900 | 58 | Inquiry |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | China | 39916 | 58 | Inquiry |
804-30-8(Fursultiamine)Related Search:
1of4
chevron_right




