Mercaptodimethur
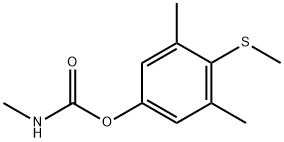
- CAS No.
- 2032-65-7
- Chemical Name:
- Mercaptodimethur
- Synonyms
- METHIOCARB;MXMC;Club;OMS93;H 321;Draza;b37344;certan;dcr736;Esurol
- CBNumber:
- CB1254073
- Molecular Formula:
- C11H15NO2S
- Molecular Weight:
- 225.31
- MOL File:
- 2032-65-7.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/6/18 13:26:42
| Melting point | 119°C |
|---|---|
| Density | 1.1838 (rough estimate) |
| vapor pressure | 1.5 x 10-5 Pa (20 °C) |
| refractive index | 1.4790 (estimate) |
| storage temp. | 0-6°C |
| solubility | DMF: 20 mg/ml,DMSO: 20 mg/ml,DMSO:PBS (pH 7.2) (1:1): 0.5 mg/ml,Ethanol: 10 mg/ml |
| form | Crystalline Solid |
| Boiling point | 310.7±42.0 °C(Predicted) |
| pka | 12.16±0.46(Predicted) |
| color | White |
| Water Solubility | Insoluble |
| BRN | 1881431 |
| Stability | Light Sensitive |
| CAS DataBase Reference | 2032-65-7(CAS DataBase Reference) |
| NIST Chemistry Reference | 4-Methylthio-3,5-xylyl methylcarbamate(2032-65-7) |
| EPA Substance Registry System | Methiocarb (2032-65-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS06,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H300-H410 | |||||||||
| Precautionary statements | P264-P270-P273-P301+P310-P391-P405 | |||||||||
| Hazard Codes | T;N,N,T | |||||||||
| Risk Statements | 25-50/53 | |||||||||
| Safety Statements | 22-37-45-60-61 | |||||||||
| RIDADR | UN 2811/2757 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | FC5775000 | |||||||||
| HazardClass | 6.1(a) | |||||||||
| PackingGroup | II | |||||||||
| Toxicity | LD50 in male, female rats (mg/kg): 70, 60 orally (Gaines) | |||||||||
| NFPA 704 |
|
Mercaptodimethur Chemical Properties,Uses,Production
Description
Methiocarb was originally developed by Bayer as an
insecticide. The bird-repellent properties of the compound
were quickly recognized, however, and a number of
applications for bird damage management followed (42).
Methiocarb is a secondary repellent, and repellency
occurs through aversive conditioning, by which birds
that feed on treated food become sick and associate
either the food or characteristics of the food with the
discomfort (21). As a result, affected birds learn to avoid
that food item. Often the avoidance response is locationdependent.
For example, common ravens (Corvus corax)
that learn not to eat eggs at one site will still feed on
eggs at a different location (43).
Chemical Properties
Methiocarb is a colorless crystalline powder.
Uses
Insecticide; molluscicide; bird repellent.
Definition
ChEBI: A carbamate ester obtained by the formal condensation of the phenolic group of 3,5-dimethyl-4-(methylsulfanyl)phenol with the carboxy group of methylcarbamic acid.
General Description
White crystalline powder with a mild odor. Used as an insecticide and immobilizing agent for birds, acaricide and molluscicide.
Reactivity Profile
Mercaptodimethur is a carbamate ester. Carbamates are chemically similar to, but more reactive than amides. Like amides they form polymers such as polyurethane resins. Carbamates are incompatible with strong acids and bases, and especially incompatible with strong reducing agents such as hydrides. Flammable gaseous hydrogen is produced by the combination of active metals or nitrides with carbamates. Strongly oxidizing acids, peroxides, and hydroperoxides are incompatible with carbamates.
Health Hazard
As a carbamate insecticide, Mercaptodimethur is a reversible cholinesterase inhibitor and acts on the nervous system. It is classified as very toxic, and the probable oral lethal dose for humans is 50-500 mg/kg or between 1 teaspoon and 1 ounce for a 150 lb. adult.
Fire Hazard
When heated to decomposition, Mercaptodimethur emits very toxic fumes of nitrogen and sulfur oxides.
Agricultural Uses
Acaricide, Molluscicide, Insecticide: Used to control slugs and snails, soil insects and spider mites in pome fruit, stone fruit, hops, strawberries, potatoes, beets, maize, vegetables and ornamentals. Also used as seed treatment to control fruit flies on maize and bird repellant on berries and cherries. Methiocarb producers deleted all food uses from their product labels between 1989-92. It is A U.S. EPA restricted Use Pesticide (RUP) except for residential application.
Trade name
AI3-25726®; ALCO SLUB”M[C]; B 37344®; BAY 5024®; BAY 9026®; BAY 37344®; BAYER 37344®; DCR 736®; DRAZA®; DRAZA G MICROPELLETS®; H 321®; MESUROL®; METHIOCARBE®; OMS- 93®; PBI SLUG GARD®; PROVADA®; SD 9228®; SLUG-GETA®[C]
Contact allergens
Methiocarb is an insecticide or molluscicide with a cholinesterase inhibiting effect. A case of contact dermatitis was reported in a carnation grower.
Pharmacology
Methiocarb is a carbamate, and its mode of action is via the inhibition of acetylcholinesterase at synapses in the nervous system. Unlike many cholinesterase-inhibiting compounds, however, the effects of methiocarb are rapidly reversible, and the animal experiences only transitory disruption.
Safety Profile
Poison by ingestion, skin contact, and intraperitoneal routes. Used as an insecticide, molluscicide, and bird repellent. When heated to decomposition it emits very toxic fumes of NOx and SOx. See also ESTERS and CARBAMATES.
Potential Exposure
A potential danger to those involved in the manufacture, formulation, and application of this nonsystemic acaricide and insecticide.
Environmental Fate
Soil. Methiocarb was oxidized, probably by singlet oxygen, to the corresponding
sulfoxide and trace amounts (<5% yield) of sulfone when sorbed on soil and exposed to
sunlight. The photosensitized oxidation was faster in soils containing the lowest organic
carbon content (Gohre and Miller, 1986).
Plant. On and/or in bean plants, the methylthio group is rapidly oxidized to the
sulfoxide and sulfone (Abdel-Wahab et al., 1966) followed by hydrolysis yielding the
corresponding thiophenol, methylsulfoxide phenol and methylsulphonyl phenol (Har
Photolytic. When methiocarb in ethanol was irradiated by UV light, only a few
unidentified cholinesterase inhibitors were formed (Crosby et al., 1965).
Chemical/Physical. Emits toxic fumes of nitrogen and sulfur oxides when heated to
decomposition (Sax and Lewis, 1987).
Metabolic pathway
Methiocarb is oxidised to a sulfoxide and a sulfone in biological media. The resulting two carbamate esters and the parent compound are hydrolysed in soils and plants to the corresponding phenols. Hydroxylation of the N-methyl group on the carbamate function occurs in mammalian preparations in vitro.
Shipping
UN2757 Carbamate pesticides, solid, toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials. UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required
Waste Disposal
In accordance with 40CFR 165 recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform to EPA regulations governing storage, transportation, treatment, and waste disposal. Remove material with contaminated soil and place in impervious containers. May be incinerated in a pesticide incinerator at the specified temperature/dwell-time combination. Any liquids, sludges, or solid residues generated should be disposed of in accordance with all applicable federal, state, and local pollution control requirements. If appropriate incineration facilities are not available, material may be buried in a chemical waste landfill. May be amenable to biological treatment at a municipal sewage treatment plant. (Sax/DPIMR).
Mercaptodimethur Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | China | 6387 | 58 | Inquiry |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | China | 39916 | 58 | Inquiry |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | China | 49392 | 58 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29824 | 58 | Inquiry |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | China | 12446 | 58 | Inquiry |
| SIMAGCHEM CORP | +86-13806087780 | China | 17367 | 58 | Inquiry |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | United States | 19892 | 58 | Inquiry |
| LEAP CHEM CO., LTD. | +86-852-30606658 | China | 24738 | 58 | Inquiry |
| Hebei Guanlang Biotechnology Co., Ltd. | +8619930503259 | China | 18423 | 58 | Inquiry |
2032-65-7(Mercaptodimethur)Related Search:
1of4
chevron_right




