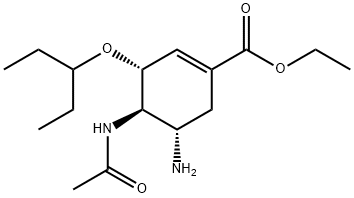
Oseltamivir
- CAS No.
- 196618-13-0
- Chemical Name:
- Oseltamivir
- Synonyms
- TAMIFLU;(3R,4R,5S)-Ethyl 4-acetamido-5-amino-3-(pentan-3-yloxy)cyclohex-1-enecarboxylate;TaMvir;GS 4104;Ostavir;GOP-A-Flu;Oseltamivr;OSELTAMIVIR;TaMiflu-Free;OSTELTAMIVIR
- CBNumber:
- CB1472402
- Molecular Formula:
- C16H28N2O4
- Molecular Weight:
- 312.4
- MOL File:
- 196618-13-0.mol
- Modify Date:
- 2024/6/24 22:20:23
| Melting point | 107-108 °C |
|---|---|
| Boiling point | 473.3±45.0 °C(Predicted) |
| Density | 1.08±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C(protect from light) |
| solubility | Chloroform (Sparingly), DMSO (Slightly), Ethyl Acetate (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 7.7 (25°); 6.6 (70°) |
| color | Off-White to Pale Beige |
| Stability | Hygroscopic |
| CAS DataBase Reference | 196618-13-0 |
| EPA Substance Registry System | 1-Cyclohexene-1-carboxylic acid, 4-(acetylamino)-5-amino-3-(1-ethylpropoxy)-, ethyl ester, (3R,4R,5S)- (196618-13-0) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H335 |
| Precautionary statements | P261-P301+P312-P302+P352-P304+P340-P305+P351+P338 |
Oseltamivir Chemical Properties,Uses,Production
Uses
Oseltamivir is an orally active inhibitor of influenza virus neuraminidase; converted in vivo to the active acid metabolite. An antiviral drug. It is a COVID19-related research product.
Definition
ChEBI: A cyclohexenecarboxylate ester that is the ethyl ester of oseltamivir acid. An antiviral prodrug (it is hydrolysed to the active free carboxylic acid in the liver), it is used to slow the spread of influenza.
Indications
Oseltamivir phosphate (Tamiflu) is the ethyl ester prodrug of oseltamivir carboxylate, an analogue of neuraminic (sialic) acid that is a reversible competitive antagonist of influenza A and B neuraminidase.Influenza virus resistant to oseltamivir has not been found in naturally acquired isolates but has been isolated from influenza patients who have undergone treatment with this drug.These resistant strains contain mutations in the active site of neuraminidase and are generally less virulent and infective than nonresistant virus. In vitro passage of influenza virus in the presence of oseltamivir carboxylate can produce mutations in hemagglutinin that decrease the overall dependence of viral replication on neuraminidase; however, the clinical relevance of this resistance mechanism is unknown.
Antimicrobial activity
Oseltamivir is active against influenza A and B, but no other virus.
Acquired resistance
Mutations in the neuraminidase (H274Y) have been detected in treated patients with seasonal H1N1 infection. Cross-resistance with zanamivir has been described in vitro.
Pharmaceutical Applications
A selective neuraminidase inhibitor, formulated as the phosphate salt of the ethyl ester for oral administration.
Pharmacokinetics
Oral absorption: c. 75%
Cmax 75 mg oral: 0.35–0.55 mg/L after 4 h
Plasma half-life: 7–9 h
Plasma protein binding: Not known
The ethyl ester prodrug is hydrolyzed by hepatic esterases
to release the active compound, oseltamivir carboxylate.
Drug is excreted in the urine as the carboxylate
derivative.
Clinical Use
Oseltamivir is approved for the treatment of uncomplicated acute influenza in patients aged 1 year and older. It decreases the duration of illness by 1 to 1.5 days when treatment is initiated within 48 hours of the onset of symptoms. Oseltamivir is also indicated for the prophylaxis of influenza in individuals aged 13 and older. It reduces infection rates to approximately 10 to 25% of that found in untreated populations; however, it is not intended to substitute for the early vaccination recommended by the CDC. Oseltamivir can be used as postexposure prophylaxis in household contacts of infected patients, with infection rates of treated patients around 10% of placebo control levels.
Side effects
The most frequently reported adverse effects of oseltamivir are nausea and vomiting.These events are usually mild to moderate, occur during the first 1 to 2 days of treatment, and can be lessened by taking the drug with food. Bronchitis, insomnia, and vertigo may also occur. Oseltamivir may not be indicated for use in certain individuals. Its efficacy in patients with chronic cardiac or respiratory disease has not been established. In clinical trials, no difference in the incidence of complications was seen between treatment and control groups. The efficacy of oseltamivir has not been demonstrated in immunocompromised patients, patients who begin treatment after 40 hours of symptoms, or patients given repeated prophylactic courses of therapy. Dosage adjustment is recommended for individuals with renal insufficiency; the drug’s safety in patients with hepatic insufficiency is unknown.
Oseltamivir Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| J S LABS | +91-7330612784 +91-7330612784 | Tamil Nadu, India | 160 | 58 | Inquiry |
| Venkatasai Life Sciences | +91-9908134868 +91-8008303069 | Hyderabad, India | 186 | 58 | Inquiry |
| Ralington Pharma | +91-7948911722 +91-9687771722 | Gujarat, India | 1350 | 58 | Inquiry |
| Sibram Pharmaceutical | +91-8655777550 +91-8655777550 | Maharashtra, India | 244 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| Aspen Biopharma Labs Private Limited | 08047620755 | Hyderabad, India | 17 | 58 | Inquiry |
| CHEMSWORTH | +91-261-2397244 | New Delhi, India | 6707 | 30 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Hebei Duling International Trade Co. LTD | +8617333973358 | China | 15438 | 58 | Inquiry |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | China | 29798 | 60 | Inquiry |
Related articles
- Uses and mechanism of Oseltamivir
- Oseltamivir is the prodrug of oseltamivir carboxylate, a virustatic inhibitor of influenza A and B virus replication. Oseltami....
- Apr 11,2022
196618-13-0(Oseltamivir)Related Search:
1of4
chevron_right




