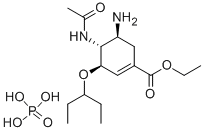
Oseltamivir phosphate
- CAS No.
- 204255-11-8
- Chemical Name:
- Oseltamivir phosphate
- Synonyms
- Osteltamivir phosphate;Oseltamivir Phosphate (200 mg);(3R,5S)-ethyl 4-acetamido-5-amino-3-(pentan-3-yloxy)cyclohex-1-enecarboxylate phosphate;(3R,4R,5S)-ethyl 4-acetaMido-5-aMino-3-(pentan-3-yloxy)cyclohex-1-enecarboxylate phosphate;SOT;Oselt;CS-1978;Oseltamivir PH;Ostavir phosphate;US-DMF No.: 035876
- CBNumber:
- CB2119221
- Molecular Formula:
- C16H31N2O8P
- Molecular Weight:
- 410.4
- MOL File:
- 204255-11-8.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/6/24 22:20:23
| Melting point | 196-198°C |
|---|---|
| storage temp. | 2-8°C |
| solubility | H2O: soluble30mg/mL, clear |
| form | powder |
| color | white to beige |
| optical activity | [α]/D -26 to -36°, c = 1 in H2O |
| Water Solubility | Soluble in water (75 mM) |
| BCS Class | 1 (CLogP), 3 (LogP) |
| InChI | InChI=1/C16H28N2O4.H3O4P/c1-5-12(6-2)22-14-9-11(16(20)21-7-3)8-13(17)15(14)18-10(4)19;1-5(2,3)4/h9,12-15H,5-8,17H2,1-4H3,(H,18,19);(H3,1,2,3,4)/t13-,14+,15+;/s3 |
| InChIKey | PGZUMBJQJWIWGJ-IFAKAUOZSA-N |
| SMILES | [C@@H]1(OC(CC)CC)C=C(C[C@H](N)[C@H]1NC(=O)C)C(=O)OCC.OP(O)(O)=O |&1:0,10,12,r| |
| CAS DataBase Reference | 204255-11-8(CAS DataBase Reference) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H317-H319-H412 | |||||||||
| Precautionary statements | P261-P264-P273-P280-P302+P352-P305+P351+P338 | |||||||||
| HazardClass | IRRITANT | |||||||||
| HS Code | 2924299500 | |||||||||
| NFPA 704 |
|
Oseltamivir phosphate price More Price(2)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | SML1606 | Oseltamivir phosphate ≥98% (HPLC) | 204255-11-8 | 100MG | ₹8486.8 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | PHR1781 | Oseltamivir Phosphate Pharmaceutical Secondary Standard; Certified Reference Material | 204255-11-8 | 500MG | ₹16215.85 | 2022-06-14 | Buy |
Oseltamivir phosphate Chemical Properties,Uses,Production
Description
Oseltamivir phosphate (Tamiflu) was launched in the US and Switzerland for the treatment of influenza infections by all common strain viruses. It is an oral anti-viral drug approved for the treatment of acute, uncomplicated influenza in patients 2 weeks of age and older whose flu symptoms have not lasted more than two days. This product is approved to treat Type A and B influenza; however, the majority of patients included in the studies were infected with type A, the most common in the U.S. Efficacy of Tamiflu in the treatment of influenza in subjects with chronic cardiac disease and/or respiratory disease has not been established.
Chemical Properties
White Cyrstalline Solid. It is freely soluble in water.
Uses
Oseltamivir phosphate (Tamiflu) is a competitive neuraminidase inhibitor. The prodrug oseltamivir phosphate (Tamiflu) is itself not virally effective; however, once in the liver, it is converted by natural chemical processes, hydrolysed hepatically to its
Definition
ChEBI: Oseltamivir phosphate is a phosphate salt. It contains an oseltamivir. It is an acetamido cyclohexene that is a structural homolog of SIALIC ACID and inhibits NEURAMINIDASE.
Preparation
Oseltamivir phosphate (Tamiflu) has been synthesized from cis-2,3-bis(hydroxymethyl)aziridine. After protection of the cis-2,3-bis(hydroxymethyl)aziridine with a Boc group, desymmetrization provided a chiral aziridine, which was a key intermediate to install the required stereogenic center containing a nitrogen atom. Allylation and ring closing metathesis are the key reactions to obtain the cyclic product that was successfully converted to the desired oseltamivir phosphate. DOI: 10.1021/jo3015853
It can also be obtained by a novel 12-step synthesis from (-)-quinic acid.
General Description
receptor site showed clearly that additional binding sitesexist for the C-5 acetamido carbonyl group and the arginineresidue at position 152 of the receptor site. In addition, the C-2 carboxyl group of sialic acid binds to Arg 118, Arg 292, andArg 371. Position C-6 is capable of undergoing a hydrophobicinteraction with various amino acids, including Glu, Ala,Arg, and Ile. Maximum binding to neuraminidase occurswhen the C-6 substituent is substituted with a nonpolar chain.In oseltamivir, this nonpolar group is 3-pentyl. An importantfeature of oseltamivir is the ethyl ester, which makes the drugorally efficacious. This drug is the first orally active agent foruse against influenza A and B. It is also indicated for the treatmentof acute illness. If administered within 2 days after theonset of influenza symptoms, the drug is effective.
Clinical Use
Oseltamivir was approved as the first orally administered neuraminidase inhibitor used against influenza A and B viruses. The drug is indicated for the treatment of uncomplicated acute illness caused by influenza infection.
Side effects
Side effects with oseltamivir are minor, consist of nausea and vomiting, and occur primarily in the first two days of therapy.
Metabolism
Oseltamivir is readily absorbed from the GI tract following oral administration. It is a prodrug that is extensively metabolized in the liver, undergoing ester hydrolysis to the active carboxylic acid. Two oxidative metabolites also have been isolated, with the major oxidation product being the ω-carboxylic acid.
Mode of action
Oseltamivir Phosphate is the phosphate salt of oseltamivir, a synthetic derivative prodrug of ethyl ester with antiviral activity. By blocking neuraminidases on the surfaces of influenza viruses, oseltamivir interferes with host cell release of complete viral particles.
Oseltamivir phosphate Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| SGMR PHARMACEUTICALS PVT LTD | +91-9032001889 +91-9032001889 | Telangana, India | 83 | 58 | Inquiry |
| SEUTIC | +91-8309787199 +91-8309787199 | Hyderabad, India | 124 | 58 | Inquiry |
| CDYMAX | +91-9019317788 +91-9019317788 | Bangalore, India | 28 | 58 | Inquiry |
| Gonane Pharma | +91-9819380043 +91-9819380043 | NaviMumbai, India | 192 | 58 | Inquiry |
| Lupin Ltd | +91-8019896181 +91-8019896181 | Maharashtra, India | 93 | 58 | Inquiry |
| Cipla Ltd | +912224826000 | Maharashtra, India | 133 | 58 | Inquiry |
| Hetero Drugs Limited | +91-4023704923 +91-4023704923 | Telangana, India | 296 | 58 | Inquiry |
| Everest Organics Limited | +91-4040028838 +91-9440409996 | Hyderabad, India | 61 | 58 | Inquiry |
| Solara Active Pharma Sciences Ltd | +91-8046632100 +91-7075706520 | Bangalore, India | 65 | 58 | Inquiry |
| Zydus Lifesciences Ltd. | +91-0265-2315243 +91-6358895661 | Gujarat, India | 89 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| SGMR PHARMACEUTICALS PVT LTD | 58 |
| SEUTIC | 58 |
| CDYMAX | 58 |
| Gonane Pharma | 58 |
| Lupin Ltd | 58 |
| Cipla Ltd | 58 |
| Hetero Drugs Limited | 58 |
| Everest Organics Limited | 58 |
| Solara Active Pharma Sciences Ltd | 58 |
| Zydus Lifesciences Ltd. | 58 |
Related articles
- Introduction, Synthesis, and Pharmacokinetics of Oseltamivir Phosphate
- Oseltamivir phosphate is an anti-flu drug marketed as Tamiflu. Appearing as A white to yellowish-white powder, it can be class....
- Jul 13,2022
204255-11-8(Oseltamivir phosphate)Related Search:
1of4
chevron_right




