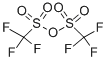
Trifluoromethanesulfonic anhydride
- CAS No.
- 358-23-6
- Chemical Name:
- Trifluoromethanesulfonic anhydride
- Synonyms
- Tf2O;TRIFLIC ANHYDRIDE;Trifluoromethylsulfonic anhydride;trifluoromethylsulfonyl trifluoromethanesulfonate;Triflate Anhydride;TRIFLUOROMETHANESULPHONIC ANHYDRIDE;TRIFLUOROMETHANESULFONIC ACID ANHYDRIDE;TRIFLUOROMETHANESULFONYL ANHYDRIDE;Triflic acid anhydride;Trifluoromethylsulfonic acid anhydride
- CBNumber:
- CB5273333
- Molecular Formula:
- C2F6O5S2
- Molecular Weight:
- 282.14
- MOL File:
- 358-23-6.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/5/15 13:45:27
| Melting point | -80°C |
|---|---|
| Boiling point | 81-83 °C (lit.) |
| Density | 1.677 g/mL at 25 °C (lit.) |
| vapor density | 5.2 (vs air) |
| vapor pressure | 8 mm Hg ( 20 °C) |
| refractive index |
n |
| RTECS | PB2772000 |
| Flash point | 81-83°C |
| storage temp. | Store below +30°C. |
| solubility | Miscible with dichloromethane. Immiscible with hydrocarbons. |
| form | Liquid |
| Specific Gravity | 1.677 |
| color | Clear colorless to light brown |
| Water Solubility | reacts violently with water |
| Sensitive | Moisture Sensitive |
| BRN | 1813600 |
| Stability | Hygroscopic, Moisture Sensitive |
| InChIKey | WJKHJLXJJJATHN-UHFFFAOYSA-N |
| LogP | 0.3 at 25℃ |
| CAS DataBase Reference | 358-23-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Trifluoromethanesulfonic anhydride(358-23-6) |
| EPA Substance Registry System | Methanesulfonic acid, trifluoro-, anhydride (358-23-6) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS03,GHS05,GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H272-H302-H314-H335 | |||||||||
| Precautionary statements | P210-P220-P280-P301+P312-P303+P361+P353-P305+P351+P338 | |||||||||
| Hazard Codes | C | |||||||||
| Risk Statements | 14-21/22-34-35-22-40 | |||||||||
| Safety Statements | 26-36/37/39-43-45-8 | |||||||||
| RIDADR | UN 3265 8/PG 2 | |||||||||
| WGK Germany | 3 | |||||||||
| F | 10-21 | |||||||||
| Hazard Note | Corrosive | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | I | |||||||||
| HS Code | 29049020 | |||||||||
| Toxicity | LD50 orally in Rabbit: 1012 mg/kg | |||||||||
| NFPA 704 |
|
Trifluoromethanesulfonic anhydride price More Price(21)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 91737 | Trifluoromethanesulfonic anhydride purum, ≥98.0% (T) | 358-23-6 | 5ML | ₹6256.15 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 91737 | Trifluoromethanesulfonic anhydride purum, ≥98.0% (T) | 358-23-6 | 25ML | ₹8144.6 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 91737 | Trifluoromethanesulfonic anhydride purum, ≥98.0% (T) | 358-23-6 | 100ML | ₹29190 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 8.18043 | Trifluoromethanesulfonic anhydride for synthesis | 358-23-6 | 2ML | ₹2260 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 176176 | Trifluoromethanesulfonic anhydride ≥99% | 358-23-6 | 5G | ₹2321 | 2022-06-14 | Buy |
Trifluoromethanesulfonic anhydride Chemical Properties,Uses,Production
Description
Trifluoromethanesulfonic anhydride, also known as triflic anhydride, has proven to be an extraordinary reagent for a broad range of transformations. As a commercially and readily available reagent, It has been widely used in synthetic chemistry due to its high electrophilicity. Given its high affinity towards O-nucleophiles, reaction with alcohols, carbonyls, sulfur phosphorus- and iodine oxides towards the formation of the corresponding triflates is strongly favored. As one of the premier leaving groups in organic chemistry, the generated triflates then open the door to various downstream transformations, including (but not limited to) substitution reactions, cross-coupling processes, redox reactions, and rearrangements[1-2].
Chemical Properties
clear colorless to light brown liquid
Uses
Trifluoromethanesulfonic Anhydride is a strong electrophile used in chemical synthesis for introducing the triflyl group.
Reactivity Profile
Electrophilic activation of tertiary and secondary amides with triflic anhydride (Tf2O; rifluoromethanesulfonic anhydride) under mild conditions gives rise to iminium and imino triflates, respectively, which could be utilized as versatile reagents to react with various C-, N-, O- and S-nucleophiles for the transformation of an amide function into different products. Other oxygen-containing nucleophiles, such as sulfoxides and phosphorus oxides, could also undergo nucleophilic attack with Tf2O to generate thionium triflate, electrophilic P-species, and phosphonium triflate. These highly active transient species could readily undergo nucleophilic substitution reactions for further diverse transformations. Furthermore, owing to the strong electrophilic property, Tf2O is prone to react with relatively weak nucleophiles such as nitrile groups or some nitrogen-containing heterocyclic compounds. In addition, Tf2O has also been used as an efficient radical trifluoromethylation and trifluorometh ylthiolation reagent through the release of SO2 or deoxygenation process for the synthesis of trifluoromethylated and trifluoromethylthiolated compounds[2].
Hazard
May be corrosive to metals. Harmful if swallowed. Causes severe skin burns and eye damage.
Synthesis
The synthesis of Trifluoromethanesulfonic anhydride is as follows:
A dry, 100-ml., round-bottomed flask is charged with 36.3 g. (0.242
mole) of trifluoromethanesulfonic acid (Note 1) and 27.3 g. (0.192 mole)
of phosphorus pentoxide (Note 2). The flask is stoppered and allowed to
stand at room temperature for at least 3 hours. During this period the
reaction mixture changes from a slurry to a solid mass. The flask is
fitted with a short-path distilling head and heated first with a stream
of hot air from a heat gun and then with the flame from a small burner.
The flask is heated until no more trifluoromethanesulfonic anhydride
distills, b.p. 82–115°, yielding 28.4–31.2 g. (83–91%) of the anhydride,
a colorless liquid. Although this product is sufficiently pure for use
in the next step, the remaining acid may be removed from the anhydride
by the following procedure. A slurry of 3.2 g. of phosphorus pentoxide
in 31.2 g. of the crude anhydride is stirred at room temperature in a
stoppered flask for 18 hours. After the reaction flask has been fitted
with a short-path distilling head, it is heated with an oil bath,
yielding 0.7 g. of forerun, b.p. 74–81°, followed by 27.9 g. of the pure
trifluoromethanesulfonic acid anhydride, b.p. 81–84° .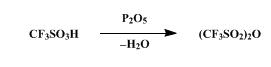
storage
Store in a cool, dry, wellventilated area. Moisture sensitive.
Purification Methods
It can be freshly prepared from the anhydrous acid (11.5g) and P2O5 (11.5g, or half this weight) by setting aside at room temperature for 1hour, distilling off volatile products then distil it through a short Vigreux column. It is readily hydrolysed by H2O and decomposes appreciably after a few days to liberate SO2 and produce a viscous liquid. Store it dry at low temperatures. [Burdon et al. J Chem Soc 2574 1957, Beard et al. J Org Chem 38 373 1973, Beilstein 3 IV 35.]
References
[1] Haoqi Zhang. “Trifluoromethanesulfonic Anhydride in Amide Activation: A Powerful Tool to Forge Heterocyclic Cores.” TCIMAIL (2021).
[2] Dr. Qixue Qin, Prof. Dr. Ning Jiao, Zengrui Cheng. “Recent Applications of Trifluoromethanesulfonic Anhydride in Organic Synthesis.” Angewandte Chemie 135 10 (2022).
Trifluoromethanesulfonic anhydride Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
1of3
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| BTC pharm India | +918790379245 | AndhraPradesh, India | 1269 | 58 | Inquiry |
| GLR Innovations | +91 9891111994 | New Delhi, India | 4542 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Mumbai, India | 66 | 58 | Inquiry |
| DAVY CHEM LABS | +91-9603460845 +91-9493444489 | Hyderabad, India | 109 | 58 | Inquiry |
| SAGAR LIFE SCIENCES PVT LTD | +91-8770359041 +91-8770359041 | Gujarat, India | 79 | 58 | Inquiry |
| ANJI BIOSCIENCES | +91-9000100077 +91-9000100077 | Hyderabad, India | 430 | 58 | Inquiry |
| SURVIVAL TECHNOLOGIES LIMITED | +91 8108285261 | Gujarat, India | 294 | 58 | Inquiry |
| LIFECHEM PHARMA | +91-9825440401 +91-9825440401 | Gujarat, India | 25 | 58 | Inquiry |
| AMINO ORGANICS | +919866989891 | Hyderabad, India | 275 | 58 | Inquiry |
| REDDY N REDDY PHARMACEUTICALS | +91-9848096759 +91-9848096759 | Hyderabad, India | 1989 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| BTC pharm India | 58 |
| GLR Innovations | 58 |
| CLEARSYNTH LABS LTD. | 58 |
| DAVY CHEM LABS | 58 |
| SAGAR LIFE SCIENCES PVT LTD | 58 |
| ANJI BIOSCIENCES | 58 |
| SURVIVAL TECHNOLOGIES LIMITED | 58 |
| LIFECHEM PHARMA | 58 |
| AMINO ORGANICS | 58 |
| REDDY N REDDY PHARMACEUTICALS | 58 |
Related articles
- A useful reagent: Trifluoromethanesulfonic anhydride
- Trifluoromethanesulfonic anhydride is employed as an electrophilic reagent for the conversion of various oxygen-containing com....
- Dec 7,2023
- Preparation of Trifluoromethanesulfonic anhydride.
- Triflic anhydride is the chemical compound with the formula (CF3SO2)2O. It is the acid anhydride derived from triflic acid. Th....
- Nov 6,2019
358-23-6(Trifluoromethanesulfonic anhydride)Related Search:
1of4
chevron_right




