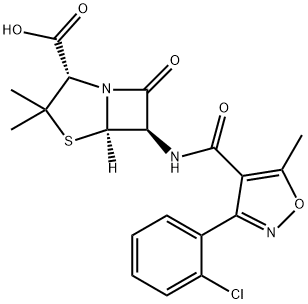
Cloxacillin
- CAS No.
- 61-72-3
- Chemical Name:
- Cloxacillin
- Synonyms
- Syntarpen;MCIPC;brl1621;CLOXACILLIN;methocillins;Cloxacillin 13C4;Cloxacillin Impurity;Orbenin Extra Dry Cow;CLOXACILLIN USP/EP/BP;Cloxacillin (base and/or unspecified salts)
- CBNumber:
- CB7163623
- Molecular Formula:
- C19H18ClN3O5S
- Molecular Weight:
- 435.88
- MOL File:
- 61-72-3.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/8/21 22:41:43
| Boiling point | 689.7±55.0 °C(Predicted) |
|---|---|
| Density | 1.2954 (rough estimate) |
| refractive index | 1.6100 (estimate) |
| pka | pKa 2.70±0.07(H2O t=35.0 c=0.0025) (Uncertain) |
| InChIKey | LQOLIRLGBULYKD-JKIFEVAISA-N |
| SMILES | N12[C@@]([H])([C@H](NC(C3=C(C)ON=C3C3=CC=CC=C3Cl)=O)C1=O)SC(C)(C)[C@@H]2C(O)=O |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|---|---|
| Signal word | Danger |
| Hazard statements | H317-H334 |
| Precautionary statements | P261-P285-P304+P341-P342+P311-P501-P261-P272-P280-P302+P352-P333+P313-P321-P363-P501 |
Cloxacillin Chemical Properties,Uses,Production
Description
Cloxacillin induced contact dermatitis in a pharmaceutical factory worker with positive reactions to ampicillin but not to penicillin. The chemical formula of this is 3-o-chlorophenyl-5-methyl-4-isoxazolyl penicillin (Knudsen et al., 1962); this differs from oxacillin only by an additional chlorine atom.
Uses
Cloxacillin is a semisynthetic beta-lactamase resistant penicillin antibiotic with antibacterial activity. It is used to treat a wide variety of bacterial infections.
Manufacturing Process
The reaction between 6-aminopenicillanic acid (6.5 g) and 3-o-chlorophenyl-5-
methylisoxazole4-carbonyl chloride (7.66 g) gave the sodium salt of 3-o-chlorophenyl-5-methyl-4-isoxazolylpenicillin(Cloxacillin) (9.98 g) as a pale yellow solid.
Colorimetric assay with hydroxylamine against a benzylpenicillin standard
indicated a purity of 68%.The 3-o-chlorophenyl-5-methylisoxazole-4-carboxylic acid, from which the acid
chloride was prepared, was obtained by hydrolysis of the ester product of the
reaction between o-chlorobenzohydroxamic chloride and ethyl acetoacetate in
methanolic sodium methoxide. Reaction with thionyl chloride gave the starting
material.
World Health Organization (WHO)
Ampicillin and cloxacillin are listed separately in the WHO Model List of Essential Drugs.
Contact allergens
Cloxacillin is a semisynthetic penicillin close to oxacillin. It induced contact dermatitis in a pharmaceutical factory worker with positive reactions to ampicillin, but not to penicillin. In cutaneous drug reactions such as acute generalized exanthematous pustulosis due to amoxicillin, cross-reactivity is frequent to cloxacillin (personal observations).
Side effects
Common side effects include Upset stomach, nausea, vomiting, diarrhea, gas, and mouth sores may occur.
Purification Methods
Purify mandelic acid by Soxhlet extraction with *C6H6 (about 6mL/g) and allow the extract to crystallise. It can also be recrystallised from CHCl3. The S-benzylisothiuronium salt has m 169o (166o) (from H2O). Dry it at room temperature under vacuum. [Beilstein 10 IV 565.]
Cloxacillin Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Medec Dragon Private Limited | +91-9768474716 | Maharashtra, India | 59 | 58 | Inquiry |
| Ebenezer Industries | Gujarat, India | 96 | 58 | Inquiry | |
| Pharma Affiliates | 172-5066494 | Haryana, India | 6761 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| Guangzhou Tosun Pharmaceutical Limited | +8618922120635 | China | 1000 | 58 | Inquiry |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | China | 12446 | 58 | Inquiry |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | China | 5866 | 58 | Inquiry |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | China | 21667 | 55 | Inquiry |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | China | 9343 | 55 | Inquiry |





