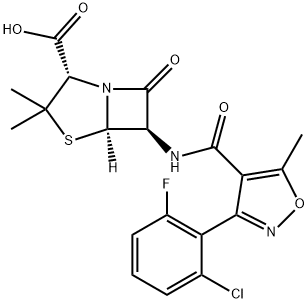
Flucloxacillin
- CAS No.
- 5250-39-5
- Chemical Name:
- Flucloxacillin
- Synonyms
- Flopen;Flucil;Culpen;FK 900;Flupen;Penplus;Floxapen;Abboflox;BRL 2039;Staphylex
- CBNumber:
- CB8690991
- Molecular Formula:
- C19H17ClFN3O5S
- Molecular Weight:
- 453.87
- MOL File:
- 5250-39-5.mol
- Modify Date:
- 2023/5/15 10:43:31
| Boiling point | 677.3±55.0 °C(Predicted) |
|---|---|
| Density | 1.59±0.1 g/cm3(Predicted) |
| solubility | soluble in Methanol, Water |
| pka | pKa 2.7 (Uncertain) |
| form | Solid |
| color | White |
| CAS DataBase Reference | 5250-39-5(CAS DataBase Reference) |
Flucloxacillin Chemical Properties,Uses,Production
Description
Chemically this is 3(2-chloro-6-fluorophenyl)-5-methyl-4-isoxazolyl penicillin; this differs from dicloxacillin only by the substitution of a fluorine for a chlorine atom (Sutherland et al., 1970). It comes as oral capsules of 250 and 500 mg, as a suspension of 25 and 50 mg/ml, and in an injectable formulation of 500 mg and 1 g.
Uses
Antibacterial.
Definition
ChEBI: A penicillin compound having a 6beta-[3-(2-chloro-6-fluorophenyl)-5-methyl-1,2-oxazole-4-carboxamido] side-chain.
Antimicrobial activity
There is complete cross-resistance with other penicillinase-stable penicillins.
General Description
Flucloxacillin was synthesized by Beecham Research Laboratories in 1962 as a penicillinase-stable and orally active semisynthetic penicillin. It shows almost the same activity as dicloxacillin, and it has slightly higher serum and tissue concentrations than dicloxacillin. This drug has been used to treat pyoderma, sepsis, and postoperative infections as well as ear and nose, respiratory tract, and other infections caused by Staphylococcus and Streptococcus, including benzylpenicillin-resistant strains.
Pharmacokinetics
Oral absorption: c. 80%
Cmax 250 mg (oral): 11 mg/L after 0.5–1 h
Plasma half-life: 2 h
Plasma protein binding: 95%
Absorption and distribution
It is well absorbed after oral administration and penetrates rapidly into extravascular exudates. Its high protein binding limits its diffusion, notably into the normal CSF.
Metabolism and excretion
Flucloxacillin is partly metabolized in the liver and about 10% of the plasma concentration is made up of metabolites. It is more slowly eliminated than cloxacillin. Some appears in the bile but about 50–80% of an oral dose is recovered from the urine, about 20% as metabolites.
Clinical Use
Uses are those of group 3 penicillins.
Side effects
In patients treated by intravenous infusion, about 5% developed phlebitis by the first and 15% by the second day, after which the proportion rose dramatically. Side effects are otherwise those common to penicillins.
Flucloxacillin Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Medec Dragon Private Limited | +91-9768474716 | Maharashtra, India | 59 | 58 | Inquiry |
| Ebenezer Industries | Gujarat, India | 96 | 58 | Inquiry | |
| PROTECH TELELINKS | +91-8571891912 +91-9855060837 | Himachal Pradesh, India | 62 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| SynZeal Research Pvt Ltd | +1 226-802-2078 | Gujarat, India | 6522 | 58 | Inquiry |
| Pharma Affiliates | 172-5066494 | Haryana, India | 6761 | 58 | Inquiry |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | China | 21669 | 55 | Inquiry |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | China | 49391 | 58 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29826 | 58 | Inquiry |
| SIMAGCHEM CORP | +86-13806087780 | China | 17367 | 58 | Inquiry |
Related articles
- Isoxazolyl Penicillins: Antimicrobial Activity, Susceptibility, Administration and Dosage Clinical Uses etc.
- These semisynthetic compounds, derived from the penicillin nucleus, 6-APA, are all closely related, being 3:5 disubstituted 4-....
- Mar 15,2022
5250-39-5(Flucloxacillin)Related Search:
1of4
chevron_right




