Lovastatin
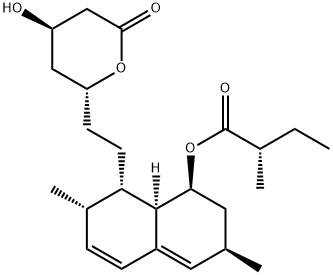
- CAS No.
- 75330-75-5
- Chemical Name:
- Lovastatin
- Synonyms
- MONACOLIN K;MEVINOLIN;LOVASTATION;Lovastatin,Monacolin K;mevlor;msd803;MK-803;Sivlor;LOVALIP;MEVACOR
- CBNumber:
- CB7169342
- Molecular Formula:
- C24H36O5
- Molecular Weight:
- 404.54
- MOL File:
- 75330-75-5.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/8/21 22:41:43
| Melting point | 175°C |
|---|---|
| alpha | D25 +323° (c = 0.5 g in 100 ml acetonitrile) |
| Boiling point | 559.2±50.0 °C(Predicted) |
| Density | 1.12±0.1 g/cm3(Predicted) |
| vapor pressure | 0Pa at 25℃ |
| refractive index | 320 ° (C=0.5, CH3CN) |
| storage temp. | -20°C |
| solubility | ethanol: soluble9.80-10.20mg/mL, clear, colorless to faintly yellow |
| form | White to off-white powder |
| pka | 13.49±0.40(Predicted) |
| color | White |
| Water Solubility | 0.0004 mg/mL at 25 ºC |
| Merck | 14,5586 |
| BCS Class | 2 |
| Stability | Stable for 2 years from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 2 months. |
| LogP | 4.260 |
| CAS DataBase Reference | 75330-75-5(CAS DataBase Reference) |
| EPA Substance Registry System | Lovastatin (75330-75-5) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H351-H361f | |||||||||
| Precautionary statements | P201-P202-P280-P308+P313-P405-P501 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 36/37/38 | |||||||||
| Safety Statements | 22-24/25-36/37/39-26 | |||||||||
| RIDADR | 3077 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | EK7907000 | |||||||||
| HazardClass | 9 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29322090 | |||||||||
| Toxicity | LD50 orally in mice: >1000 mg/kg (Endo) | |||||||||
| NFPA 704 |
|
Lovastatin price More Price(5)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | PHR1285 | Lovastatin Pharmaceutical Secondary Standard; Certified Reference Material | 75330-75-5 | 1G | ₹8042.98 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | M2147 | Mevinolin from Aspergillus sp. ≥98% (HPLC) | 75330-75-5 | 25MG | ₹22440.23 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 438185 | Lovastatin - CAS 75330-75-5 - Calbiochem Lovastatin, CAS 75330-75-5, is an anti-hypercholesterolemic agent that inhibits the activity of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reduct | 75330-75-5 | 25MG | ₹20710 | 2022-06-14 | Buy |
| TCI Chemicals (India) | L0214 | Lovastatin | 75330-75-5 | 5G | ₹9000 | 2022-05-26 | Buy |
| TCI Chemicals (India) | L0214 | Lovastatin | 75330-75-5 | 25G | ₹13200 | 2022-05-26 | Buy |
Lovastatin Chemical Properties,Uses,Production
Description
Lovastatin is an orally-active hypocholesterolemic useful in the treatment of familial hypercholesterolemia. The drug acts by inhibiting the HMG-CoA reductase-catalyzed rate limiting step of cholesterol biosynthesis. While treatment with lovastatin leads to significant reductions in total and LDL cholesterol, its effect on atherosclerosis or coronary heart disease has not been established.
Chemical Properties
White Solid
Physical properties
Appearance: White, nonhygroscopic crystalline powder. Solubility: Insoluble in water (0.0004?mg/mL); sparingly soluble in ethanol, methanol, isobutanol, isopropanol, acetonitrile, n-propanol; soluble in acetone, N-dimethylformamide; freely soluble in chloroform. Melting point: 174.5?°C. Specific optical rotation: 25?°C for D (sodium) line, +323° (concentration 0.5? g in 100? ml acetonitrile). Stability: Lovastatin is sensitive to light. Following exposure to extreme light conditions, the drug is stable for 24?h or 1?month when exposed to UV (approximately 3230 lux) or fluorescent (approximately 10,764 lux) light, respectively, at 28?°C in air.
History
Statins are the most extensively used class of lipid-lowering medication. Lovastatin
is the second statin discovered by scientists.
Later, the official name lovastatin was established. The activity of lovastatin is
much better than compactin.
In July 1982, lovastatin showed dramatic effects on lowering LDL cholesterol in
patients with severe hypercholesterolemia who were unresponsive to the existedmedicines, with very few adverse reactions .
Uses
Lovastatin (mevinolin) is a metabolite first isolated from Monascus ruber and later found in several other fungal species. Lovastatin is a potent inhibitor of HMG-CoA. HMG-CoA reductase is the rate-controlling enzyme of the mevalonate pathway, responsible for the biosynthesis of cholesterol. Lovastatin was developed as a drug as a hypolipemic agent.
Indications
Hypercholesterolemia, combined hyperlipidemia.
General Description
Lovastatin, 2-methylbutanoic acid 1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-[2-(tetrahydro-4-hydroxy6-oxo-2H-pyran-2-yl)ethyl]-1-naphthalenyl ester, mevinolin,MK-803 (Mevacor) (formerly called mevinolin), is a potentinhibitor of HMG-CoA. The drug was obtained originallyfrom the fermentation products of the fungi Aspergillus terreusand Monascus ruber. Lovastatin was one of two originalHMG-CoA reductase inhibitors. The other drug, mevastatin(formerly called compactin), was isolated from cultures ofPenicillium cillium citrum. Mevastatin was withdrawn fromclinical trials because it altered intestinal morphology in dogs.This effect was not observed with lovastatin. For inhibitoryeffects on HMG-CoA reductase, the lactone ring must be hydrolyzedto the open-ring heptanoic acid.
Biological Activity
Potent, competitive inhibitor of HMG-CoA reductase (K i = 0.6 nM) therefore decreases cholesterol biosynthesis, in vitro and in vivo . Decreases CDK2, 4, 6 and cyclin E levels and induces G1 arrest and apoptosis in tumor cell lines in vitro .
Pharmacology
Lovastatin is an inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A reductase
(HMG-CoA reductase), an enzyme that catalyzes the conversion of HMG-CoA to
mevalonate , a rate-determining step of cholesterol biosynthesis. Lovastatin is
metabolized as a prodrug into an active form, lovastatin acid. Lovastatin acid is a
reversible competitive inhibitor for HMG-CoA.
However, the reduction in plasma cholesterol by statins is not only due to reduction in cholesterol biosynthesis. .
In addition to their lipid-lowering properties, statins produce several nonlipidrelated properties, include decreasing levels of high-sensitivity C-reactive protein
(hsCRP), improving endothelial function, reducing inflammation at the site of the
atherosclerotic plaque, inhibiting platelet aggregation, anticoagulant, etc. .
Clinical Use
The primary uses of lovastatin are the treatment of dyslipidemia and the prevention of cardiovascular disease. It is recommended to be used only when other approaches such as diet, exercise, and weight reduction have not improved the cholesterol profile (“Lovastatin”. The American Society of Health-System Pharmacists. Retrieved 3 April 2011.). Lovastatin is useful in treating hypercholesterolemia and combined hyperlipidemia. However, lovastatin is not effective in treatment of receptornegative homozygous familial hypercholesterolemia .
Side effects
Lovastatin's common side effects are listed in approximately descending order of occurrence frequency: creatine phosphokinase elevation, flatulence, abdominal pain, constipation, diarrhea, muscle aches or pains, nausea, indigestion, weakness, blurred vision, rash, dizziness, and muscle cramps. As with all statin drugs, it can rarely cause myopathy, hepatotoxicity (liver damage), dermatomyositis, or rhabdomyolysis.
Lovastatin Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Concord Biotech Limited | +91-2714398200 +91-2714398200 | Gujarat, India | 22 | 58 | Inquiry |
| Ebenezer Industries | Gujarat, India | 96 | 58 | Inquiry | |
| Krebs Biochemicals And Industries Limited | +91-4066808040 +91-4066808040 | Telangana, India | 3 | 58 | Inquiry |
| Scinva Chemicals and Pharmaceuticals Pvt Ltd | +91-7406196725 +91-7406196725 | Karnataka, India | 71 | 58 | Inquiry |
| Vishrudh laboratories pvt ltd | +91-9666132889 +91-8097369002 | Telangana, India | 117 | 58 | Inquiry |
| GLP Pharma Standards | +91 9866074638 | Hyderabad, India | 1644 | 58 | Inquiry |
| SETV ASRV LLP | +91-9731133411 +91-9731133411 | Telangana, India | 531 | 58 | Inquiry |
| Allmpus Laboratories Pvt Ltd | +91-9518738415 +91-9167862134 | Maharashtra, India | 657 | 58 | Inquiry |
| Pacific Agencies | 08048372547Ext 801 | Mumbai, India | 135 | 58 | Inquiry |
| Krebs Bio Chemical & Industries Limited | 09849658924 | Hyderabad, India | 2 | 58 | Inquiry |
75330-75-5(Lovastatin)Related Search:
1of4
chevron_right




