Methyllithium
- CAS No.
- 917-54-4
- Chemical Name:
- Methyllithium
- Synonyms
- MELI;LITHIUM METHANIDE;Methyllithium solution;Methyllithium lithium bromide complex solution;Lowhalide;1-2MinEther;methyl-lithiu;METHYLLITHIUM;Methy-Lithium;LITHIUM METHYL
- CBNumber:
- CB7234211
- Molecular Formula:
- CH3Li
- Molecular Weight:
- 21.98
- MOL File:
- 917-54-4.mol
- Modify Date:
- 2023/8/17 13:06:49
| Melting point | 70-71℃ |
|---|---|
| Boiling point | 88℃ |
| Density | 0.85 g/mL at 20 °C |
| vapor density | 3 (vs air) |
| Flash point | 5 °F |
| storage temp. | 2-8°C |
| form | Solution |
| color | Colorless to yellow |
| Specific Gravity | 0.83 |
| Water Solubility | Reacts with water. |
| Sensitive | Air & Moisture Sensitive |
| Hydrolytic Sensitivity | 9: reacts extremely rapidly with atmospheric moisture - may be pyrophoric - glove box or sealed system required |
| BRN | 3587162 |
| Exposure limits |
ACGIH: TWA 400 ppm; STEL 500 ppm OSHA: TWA 400 ppm(1200 mg/m3) NIOSH: IDLH 1900 ppm |
| CAS DataBase Reference | 917-54-4(CAS DataBase Reference) |
| EPA Substance Registry System | Lithium, methyl- (917-54-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |       GHS02,GHS05,GHS07,GHS08,GHS09,GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H252-H303-H315-H318-H401-H225-H260-H304-H314-H335-H411-H250-H302-H336-H311 | |||||||||
| Precautionary statements | P210-P223-P231+P232-P261-P370+P378-P422-P222-P280-P305+P351+P338-P309-P310-P402+P404-P301+P310a-P303+P361+P353-P405-P501a | |||||||||
| Hazard Codes | F+,C,N,F,Xn,Xi | |||||||||
| Risk Statements | 12-15-17-22-34-66-67-65-51/53-11-36/37/38-36/38-19-40-36/37-37-14/15 | |||||||||
| Safety Statements | 16-26-36/37/39-45-43-30-60-62-61-36/37 | |||||||||
| RIDADR | UN 3399 4.3/PG 1 | |||||||||
| WGK Germany | 2 | |||||||||
| F | 10 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 4.3 | |||||||||
| PackingGroup | I | |||||||||
| HS Code | 29319090 | |||||||||
| NFPA 704 |
|
Methyllithium price More Price(7)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 514330 | Methyllithium solution 3.1?M in diethoxymethane | 917-54-4 | 100ML | ₹10933.25 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 514330 | Methyllithium solution 3.1?M in diethoxymethane | 917-54-4 | 4X25ML | ₹11322.95 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 186201 | Methyllithium lithium bromide complex solution 1.5?M in diethyl ether | 917-54-4 | 4X25ML | ₹10338.98 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 514330 | Methyllithium solution 3.1?M in diethoxymethane | 917-54-4 | 1L | ₹64517 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 186201 | Methyllithium lithium bromide complex solution 1.5?M in diethyl ether | 917-54-4 | 100ML | ₹10899.45 | 2022-06-14 | Buy |
Methyllithium Chemical Properties,Uses,Production
Chemical Properties
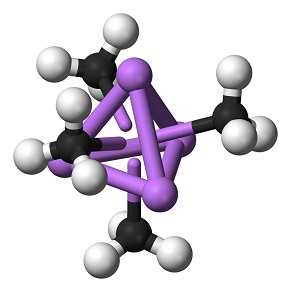
Methyllithium is a colorless to yellowish solution, It is insoluble in hydrocarbon solvents although propyllithium and the higher homologues are miscible in hydrocarbons. Methyllithium is soluble in diethyl ether to about 6% by weight at room temperature (with or without lithium bromide) and is a little less soluble in tetrahydrofuran. Cooling of these solutions produces clear, colorless crystals of methyllithium etherate. Methyllithium is stable to about 200°C, at which temperature it disproportionates to dilithiomethane and methane.
Uses
There are no significant industrial uses of methyllithium, but it is widely used in the laboratory in Grignard type reactions.
Methyllithium is an organolithium reagent mainly used for deprotonation and as a source of methyl anion. It is a strong base and a strong nucleophile.
Preparation
Methyllithium is produced industrially by the reaction of methyl chloride or methyl bromide with lithium metal dispersion in diethyl ether. The reaction with methyl chloride yields a solution of methyllithium and a precipitate of lithium chloride which may be readily separated. When methyl bromide is used, the lithium bromide byproduct remains in solution and complexes with the methyllithium. This reduces the reactivity of the methyllithium and, in certain cases, affects its usefulness. On the other hand, the less reactive complex is more stable in diethyl ether. Methyllithium produced from methyl chloride contains only about 0.3 % lithium chloride in a 5 % solution in diethyl ether. It is more reactive than the lithium bromide complex, but decomposes at the rate of 1 % of the contained methyllithium per year (that is 5.00 % solution goes to 4.95 %) at room temperature. The decomposition by ether cleavage results in methane and ethylene formation which increases the pressure in the container on long storage. Methyllithium can be prepared easily in tetrahydrofuran but it has a half-life of only about 2 days at room temperature due to solvent cleavage.
Application
Methyllithium solution (1.6M in diethyl ether) has been used for the asymmetric allylic alkylation (AAA) of allylic electrophiles in the presence of a chiral copper catalyst. This protocol leads to the C-C bond formation of tertiary and quaternary stereogenic centers with high enantioselectivity. It can also be used for the synthesis of (?)-salsolidine by reacting with 6,7-dimethoxy-3,4-dihydroisoquinoline in the presence of (?)-sparteine as a chiral ligand.
Hazard
Flammable, dangerous fire and explosionrisk, self-ignites in air.
Methyllithium Preparation Products And Raw materials
Raw materials
Preparation Products
1of4
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Almas Life Sciences | +919441456585 | Hyderabad, India | 54 | 58 | Inquiry |
| VASISTA LIFE SCIENCES PRIVATE LIMITED | +91-04029554774 +91-9010855544 | Hyderabad, India | 153 | 58 | Inquiry |
| Oceanic Pharmachem Pvt Ltd | +91-91-2242128600 +91-2242128600 | Maharashtra, India | 33 | 58 | Inquiry |
| JSK Chemicals | +919879767970 | Gujarat, India | 3756 | 58 | Inquiry |
| Ortin Laboratories Limited | +91-9241566560 +91-9241566560 | Hyderabad, India | 31 | 58 | Inquiry |
| Sanpra Synthesis Pvt. Ltd. | +91-4043460830 +91-9246161600 | AndhraPradesh, India | 49 | 58 | Inquiry |
| Organo Metallics | +91-9391016448 +91-9705345345 | Telangana, India | 32 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Alfa Aesar | 1 800 209 7001 | Maharashtra, India | 6913 | 58 | Inquiry |
| Sainor Laboratories Private Limited | 09666644167 | Hyderabad, India | 66 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| Almas Life Sciences | 58 |
| VASISTA LIFE SCIENCES PRIVATE LIMITED | 58 |
| Oceanic Pharmachem Pvt Ltd | 58 |
| JSK Chemicals | 58 |
| Ortin Laboratories Limited | 58 |
| Sanpra Synthesis Pvt. Ltd. | 58 |
| Organo Metallics | 58 |
| CLEARSYNTH LABS LTD. | 58 |
| Alfa Aesar | 58 |
| Sainor Laboratories Private Limited | 58 |





