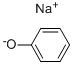
Sodium benzenolate
- CAS No.
- 139-02-6
- Chemical Name:
- Sodium benzenolate
- Synonyms
- SODIUM PHENOXIDE;SODIUM PHENATE;SODIUM PHENOLATE;Phenolsodium;PHENYL SODIUM;SODIUM PHENYL;Phenoxysodium;phenolatesodium;sodiumcarbolate;Natriumphenolat
- CBNumber:
- CB7306886
- Molecular Formula:
- C6H5NaO
- Molecular Weight:
- 116.09
- MOL File:
- 139-02-6.mol
- Modify Date:
- 2023/11/10 11:05:08
| Melting point | >300℃ |
|---|---|
| Density | 0,898 g/cm3 |
| RTECS | SM8780000 |
| Flash point | 28°C |
| storage temp. | below 5° C |
| solubility | DMSO (Slightly, Sonicated), Methanol (Slightly) |
| form | Solid |
| color | Pale Yellow to Pale Brown |
| Specific Gravity | 0.898 |
| Water Solubility | Very soluble in water, soluble in alcoholSoluble in water, acetone and alcohol. |
| Sensitive | Hygroscopic |
| Stability | Hygroscopic |
| CAS DataBase Reference | 139-02-6(CAS DataBase Reference) |
| EPA Substance Registry System | Sodium phenate (139-02-6) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS05 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H314 | |||||||||
| Precautionary statements | P260-P280-P301+P330+P331 | |||||||||
| Hazard Codes | C | |||||||||
| Risk Statements | 34 | |||||||||
| Safety Statements | 26-36/37/39-45 | |||||||||
| RIDADR | 3286 | |||||||||
| TSCA | No | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 2907110000 | |||||||||
| NFPA 704 |
|
Sodium benzenolate price More Price(4)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| ALFA India | ALF-B25002-30 | Sodium phenoxide, 98% | 139-02-6 | 250g | ₹44069 | 2022-05-26 | Buy |
| ALFA India | ALF-B25002-18 | Sodium phenoxide, 98% | 139-02-6 | 50g | ₹8046 | 2022-05-26 | Buy |
| ottokemi | S 3209 | Sodium phenoxide 98% | 139-02-6 | 50gm | ₹8604 | 2022-05-26 | Buy |
| ottokemi | S 3209 | Sodium phenoxide 98% | 139-02-6 | 250gm | ₹43902 | 2022-05-26 | Buy |
Sodium benzenolate Chemical Properties,Uses,Production
Chemical Properties
Sodium phenate, or sodium phenoxide, NaOC6H5, is a white crystalline substance that easily dissolves in water and alcohol. It decomposes when exposed to carbon dioxide in the air. It is produced by reacting sodium hydroxide solution (not carbonate) with phenol and subsequently evaporating the mixture. Sodium phenate is commonly used in the production of sodium salicylate.
Uses
As a general disinfectant, either in solution or mixed with slaked lime, etc., for toilets, stables, cesspools, floors, drains, etc.; for the manufacture of colorless or light-colored artificial resins, many medical and industrial organic Compounds and dyes; as a reagent in chemical analysis. Pharmaceutic aid (preservative).
Preparation
Sodium phenoxide was freshly prepared by adding sodium metal to phenol (0.45 g, 4.8 mmol) dissolved in diethyl ether (15 cm3), under an atmosphere of dry nitrogen. This was refluxed with (11) (1.0 g, 2.8 mmol) for 23 h. Following evaporation of the ether, water (20 cm3) was added and the mixture extracted into DCM. The DCM solution was dried (MgS04) and evaporated to give a solid (1.2 g). Fractional sublimation and recrystallisation from acetonitrile gave recovery of 2,4,6-tribromo-3,5-difluoropyridine (0.8 g, 80%) and 3-phenoxy-5-fluoro-2,4,6-tribromopyridine (0.2 g, 20%), m.p. 100±1 OC (Found: C, 31.3; H, 1.1; N, 2.9. C11H5Br3FNO requires C, 31.0; H, 1.2; N, 3.3%); IR spectrum no. 15; mass spectrum no. 15; nmr spectrum no. 19.
General Description
A white to reddish colored solid in the form of crystalline rods. Used as an antiseptic and in organic synthesis.
Air & Water Reactions
Decomposes in air. Soluble in water. In both cases, a corrosive alkaline solution forms that is a strong irritant to skin and eyes.
Reactivity Profile
Salts, basic, such as SODIUM PHENOLATE, are generally soluble in water. The resulting solutions contain moderate concentrations of hydroxide ions and have pH's greater than 7.0. They react as bases to neutralize acids. These neutralizations generate heat, but less or far less than is generated by neutralization of the bases in reactivity group 10 (Bases) and the neutralization of amines. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible.
Hazard
Strong irritant to skin and tissue.
Safety Profile
Poison by subcutaneous route. A corrosive irritant to skin, eyes, and mucous membranes. When heated to decomposition it emits toxic fumes of Na2O. See also PHENOL and SODIUM HYDROXIDE.
Purification Methods
The ground powder is washed with Et2O, then heated at 60o/1mm for 12 to 24hours to remove any free phenol and solvent. [Kornblum & Lurie J Am Chem Soc 81 2710 1959, Beilstein 6 I 718.]
Sodium benzenolate Preparation Products And Raw materials
Raw materials
Preparation Products
1of4
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Sri Neelima Laboratories | +91-7981733602 +91-9849736989 | Telangana, India | 205 | 58 | Inquiry |
| CHEMSWORTH | +91-261-2397244 | New Delhi, India | 6707 | 30 | Inquiry |
| Otto Chemie Pvt. Ltd. | +91 9820041841 | Mumbai, India | 5873 | 58 | Inquiry |
| Alfa Aesar | 1 800 209 7001 | Maharashtra, India | 6913 | 58 | Inquiry |
| Nikunj Chemicals | 08048967857 | Vadodara, India | 30 | 58 | Inquiry |
| Reax Chemicals | 08048372701Ext 416 | Hyderabad, India | 4223 | 58 | Inquiry |
| Siri Organics | 08985670323 | Hyderabad, India | 54 | 58 | Inquiry |
| Panhas Chemicals Pvt. Ltd. | 91-9586648484 | Gujarat, India | 23 | 58 | Inquiry |
| Sri Neelima Laboratories | 08048372574Ext 232 | Hyderabad, India | 154 | 58 | Inquiry |
| Kalpana Chemicals | 08068441045Ext 804 | Ahmedabad, India | 81 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| Sri Neelima Laboratories | 58 |
| CHEMSWORTH | 30 |
| Otto Chemie Pvt. Ltd. | 58 |
| Alfa Aesar | 58 |
| Nikunj Chemicals | 58 |
| Reax Chemicals | 58 |
| Siri Organics | 58 |
| Panhas Chemicals Pvt. Ltd. | 58 |
| Sri Neelima Laboratories | 58 |
| Kalpana Chemicals | 58 |
139-02-6(Sodium benzenolate)Related Search:
1of4
chevron_right




