Sodium hydrogen difluoride
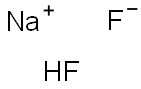
- CAS No.
- 1333-83-1
- Chemical Name:
- Sodium hydrogen difluoride
- Synonyms
- SODIUM DIFLUORIDE;SODIUM BIFLUORIDE;SodiuM hbifluoride;sodium acid fluride;fluoruroacidodesodio;Sodium hydrogen difl;SODIUM ACID FLUORIDE;Natriumhydrogenfluorid;sodiumbifluoride,solid;sodiumfluoride(na(hf2))
- CBNumber:
- CB7670064
- Molecular Formula:
- F2HNa
- Molecular Weight:
- 61.99
- MOL File:
- 1333-83-1.mol
- MSDS File:
- SDS
- Modify Date:
- 2025/1/27 9:38:02
| Melting point | >68 °C (dec.) |
|---|---|
| Boiling point | 270℃[at 101 325 Pa] |
| Density | 2.08 |
| vapor pressure | 0.01Pa at 20℃ |
| form | pellets |
| color | white hexagonal, hexane crystals, crystalline |
| Water Solubility | g/100g solution in H2O: 3.7 (20°C), 16.4 (80°C) [KIR78] |
| Merck | 13,8656 |
| LogP | 1.26 at 20℃ |
| CAS DataBase Reference | 1333-83-1(CAS DataBase Reference) |
| EPA Substance Registry System | Sodium bifluoride (1333-83-1) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS05,GHS06 |
|---|---|
| Signal word | Danger |
| Hazard statements | H301-H314 |
| Precautionary statements | P260-P280-P301+P310+P330-P301+P330+P331-P303+P361+P353-P305+P351+P338+P310 |
| Hazard Codes | T,C |
| Risk Statements | 25-34 |
| Safety Statements | 22-26-37-45 |
| RIDADR | UN 2439 8/PG 2 |
| WGK Germany | 1 |
| RTECS | WB0350010 |
| HazardClass | 8 |
| PackingGroup | II |
| HS Code | 2826199090 |
| Hazardous Substances Data | 1333-83-1(Hazardous Substances Data) |
Sodium hydrogen difluoride price More Price(4)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 437646 | Sodium hydrogen difluoride granular, 98% | 1333-83-1 | 1KG | ₹5358.38 | 2022-06-14 | Buy |
| ottokemi | S 1678 | Sodium bifluoride 98% | 1333-83-1 | 500gm | ₹1341 | 2022-05-26 | Buy |
| ottokemi | S 1678 | Sodium bifluoride 98% | 1333-83-1 | 5kg | ₹11079 | 2022-05-26 | Buy |
| SRL | 89658 | Sodium Bifluoride pure, 98% | 1333-83-1 | 500Gms | ₹1430 | 2022-05-26 | Buy |
Sodium hydrogen difluoride Chemical Properties,Uses,Production
Chemical Properties
White, crystalline powder.Sol- uble in water; decomposes on heating.
Uses
As a "sour" in laundering.
General Description
Sodium bifluoride is a white crystalline solid. Sodium hydrogen difluoride is soluble in water. Sodium hydrogen difluoride is corrosive to tissue. Sodium hydrogen difluoride is used as a preservative for anatomical and zoological specimens, in metal plating and for many other uses.
Air & Water Reactions
Water soluble. Reacts with water liberating heat and forming a corrosive solution. The reaction is not violent.
Reactivity Profile
Acidic salts, such as SODIUM BIFLUORIDE, are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Many of these compounds catalyze organic reactions.
Hazard
Highly toxic, strong irritant to tissue.
Health Hazard
INHALATION OF DUST: Irritating and possibly corrosive to mucous membranes. EYES: Irritating. INGESTION: Salty or soapy taste, salivation, nausea, burning or crampy abdominal pain, vomiting, diarrhea, muscle weakness, tremors. Rare: transient epileptiform convulsions, followed by CNS depression. Shock.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Safety Profile
This material is very toxic to humans by ingestion; between 1 teaspoonful and 1 ounce may be fatal. Inhalation of dust may cause irritation to respiratory tract. Skin contact may result in irritation and ulceration; eye contact may cause burns. To fight fire, use water, foam, CO2, dry chemicals. When heated to decomposition it emits toxic fumes of Fand Na2O. See also FLUORIDES and HYDROFLUORIC ACID.
Sodium hydrogen difluoride Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Jayfluoride PVT LTD | +91-8128987973 +91-6356977002 | Gujarat, India | 27 | 58 | Inquiry |
| ARRAKIS INDUSTRIES LLP | +91 74995 32711 | Maharashtra, India | 1353 | 58 | Inquiry |
| CHEMSWORTH | +91-261-2397244 | New Delhi, India | 6700 | 30 | Inquiry |
| Otto Chemie Pvt. Ltd. | +91 9820041841 | Mumbai, India | 5870 | 58 | Inquiry |
| Sisco Research Laboratories Pvt. Ltd. | +91-22-4268 5800 | Mumbai, India | 4315 | 58 | Inquiry |
| Triveni chemicals | 08048762458 | New Delhi, India | 6088 | 58 | Inquiry |
| Awishkar Chemical Industries | 08046060359 | Vadodara, India | 9 | 58 | Inquiry |
| Parshwamani Metals | 08046061515 | Mumbai, India | 67 | 58 | Inquiry |
| M/s Shiv Traders | 08048372740Ext 225 | Ahmedabad, India | 18 | 58 | Inquiry |
| Maa Saptashrungi Chemicals | 07942562836 | Mumbai, India | 33 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| Jayfluoride PVT LTD | 58 |
| ARRAKIS INDUSTRIES LLP | 58 |
| CHEMSWORTH | 30 |
| Otto Chemie Pvt. Ltd. | 58 |
| Sisco Research Laboratories Pvt. Ltd. | 58 |
| Triveni chemicals | 58 |
| Awishkar Chemical Industries | 58 |
| Parshwamani Metals | 58 |
| M/s Shiv Traders | 58 |
| Maa Saptashrungi Chemicals | 58 |
1333-83-1(Sodium hydrogen difluoride)Related Search:
1of4
chevron_right




