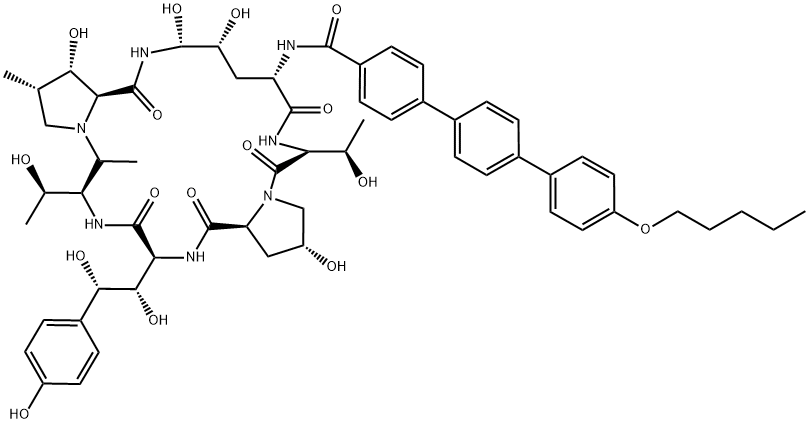
Anidulafungin
|
- ₹0
- Product name: Anidulafungin
- CAS: 166663-25-8
- MF: C58H73N7O17
- MW: 1140.24
- EINECS:658-060-4
- MDL Number:MFCD00917070
- Synonyms:Anidulafungin;Echinocandin B, 1-(4R,5R)-4,5-dihydroxy-N2-4-(pentyloxy)1,1:4,1-terphenyl-4-ylcarbonyl-L-ornithine-;Anidulafungin(LY303366);Ecalta;LY 303366;LY 303366, Eraxis;1-[(4R,5R)-4,5-dihydroxy-N2-[[4''-(pentyloxy)[1,1'',1''-terphenyl]-4-yl]carbonyl]-L-ornithine]-echinocandin B;Anidulafungin-d11
| Manufacturer | Product number | Product description | Packaging | Price | Updated | Buy |
|---|
Properties
Melting point :>196°C (subl.)
Boiling point :1477.0±65.0 °C(Predicted)
Density :1.47±0.1 g/cm3(Predicted)
storage temp. :under inert gas (nitrogen or Argon) at 2-8°C
solubility :DMSO (Slightly, Heated), Methanol (Slightly)
pka :9.86±0.26(Predicted)
form :Solid
color :White to Pale Beige
Boiling point :1477.0±65.0 °C(Predicted)
Density :1.47±0.1 g/cm3(Predicted)
storage temp. :under inert gas (nitrogen or Argon) at 2-8°C
solubility :DMSO (Slightly, Heated), Methanol (Slightly)
pka :9.86±0.26(Predicted)
form :Solid
color :White to Pale Beige
Safety Information
| Symbol(GHS): |
 
|
||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Signal word: | Warning | ||||||||||||||||||||||||||||
| Hazard statements: |
|
||||||||||||||||||||||||||||
| Precautionary statements: |
|
Description
Anidulafungin, a semi-synthetic derivative of echinocandin B, has been developed and launched as an intravenous treatment for serious fungal infections, such as candidemia, Candida-derived peritonitis, intra-abdominal abscesses, and esophageal candidiasis. As a non-competitive inhibitor of 1,3-b-D-glucan synthase, which is responsible for the formation of glucan polymers, anidulafungin interferes with the cell wall synthesis of most pathogenic fungi. This mode of action is characteristic of the echinocandin class of antifungals. While the first member of this class, cilofungin, was withdrawn due to toxicity associated with the formulation vehicle, anidulafungin follows the successful introduction of caspofungin and micafungin. Compared to the other echinocandins, anidulafungin appears to be more potent (MIC90 ofr0.25 mg/mL for C.albicans, 0.5 mg/mL for C.glabrata, 1 mg/mL for C. krusel and C.tropicalis, 2mg/mL for C.lusitaniae, and 2 mg/mL for Aspergillus spp) and is devoid of significant drug interactions since it is neither an inhibitor nor substrate of the cytochrome P450 isoenzymes. The emergence of the echinocandins circumvents the concern regarding the rising resistance to the azole and amphotericin B antifungals; no cross-resistance is expected because the echinocandins work at the cell wall rather than the cell membrane.Related product price
- Aniracetam
₹5600-12940 - 8-AZAGUANINE
₹4995 - 440-17-5
₹6800-16421.53






