GSK1349572
|
- ₹0
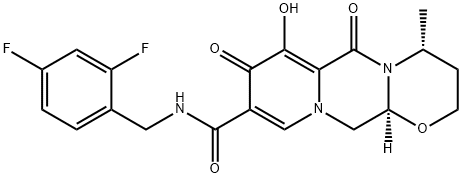
- Product name: GSK1349572
- CAS: 1051375-16-6
- MF: C20H19F2N3O5
- MW: 419.38
- EINECS:
- MDL Number:MFCD20488027
- Synonyms:Dolutegravir API;2H-Pyrido[1',2':4,5]pyrazino[2,1-b][1,3]oxazine-9-carboxamide, N-[(2,4-difluorophenyl)methyl]-3,4,6,8,12,12a-hexahydro-7-hydroxy-4-methyl-6,8-dioxo-, (4R,12aS)-;(4R,12aS)-N-[(2,4-Difluorophenyl)methyl]-3,4,6,8,12,12a-hexahydro-7-hydroxy-4-methyl-6,8-dioxo-2H-pyrido[1',2':4,5]pyrazino[2,1-b][1,3]oxazine-9-carboxamide Dolutegravir (GSK1349572);GSK1349572, S-349572, GSK572;(4R,12aS)-N-(2,4-Difluorobenzyl)-7-hydroxy-4-methyl-6,8-dioxo-3,4,6,8,12,12a-hexahydro-2H-pyrido[1',2':4,5]pyrazino[2,1-b][1,3]oxazine-9-carboxamide;Dolutegravir, >=98%;Dolutegravir free acid;GSK1349572
| Manufacturer | Product number | Product description | Packaging | Price | Updated | Buy |
|---|
Properties
Melting point :187-189°C
Boiling point :669.0±55.0 °C(Predicted)
Density :1.53
storage temp. :Refrigerator
solubility :DMSO (Slightly, Heated, Sonicated), Methanol (Slightly, Heated, Sonicated)
pka :4.50±1.00(Predicted)
form :Solid
color :White to Pale Beige
Boiling point :669.0±55.0 °C(Predicted)
Density :1.53
storage temp. :Refrigerator
solubility :DMSO (Slightly, Heated, Sonicated), Methanol (Slightly, Heated, Sonicated)
pka :4.50±1.00(Predicted)
form :Solid
color :White to Pale Beige
Safety Information
| Symbol(GHS): |

|
|||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Signal word: | Warning | |||||||||||||||||||||||||||||||||||
| Hazard statements: |
|
|||||||||||||||||||||||||||||||||||
| Precautionary statements: |
|
Description
In August 2013, the US FDA approved dolutegravir (also referred to as S/GSK1349572) for the treatment of HIV-1 infection in adults and children ages 12 years and older in combination with other antiretroviral drugs. Dolutegravir was approved in Canada in November 2013. HIV/AIDS remains a global epidemic with 35 million people infected, including 2.3 million new infections as of 2012. Dolutegravir joins raltegravir and elvitegravir (this chapter of ARMC) as the latest of three FDA-approved HIV integrase strand transfer inhibitors (INSTIs). Dolutegravir was discovered by rational design from a literature diketo acid HIV integrase inhibitor utilizing X-ray coordinates to predict ideal bond angles between the diketone and distal benzyl group. In dolutegravir, the monocyclic component of the reported inhibitor was replaced with the tricyclic carbamoyl pyridone moiety. The researchers postulated that the appropriate arrangement of three oxygens would permit chelation with two magnesium ions in the binding site thus affording improved potency. Ultimately, this arrangement along with further modifications afforded dolutegravir, a potent inhibitor of HIV integrase (IC50=1.7 nM).Related product price
- Posaconazole
₹3600-53854.38 - Boron carbide
₹1089-76509 - DL-Malic acid
₹891-8991






